First Principles Study on Electronic Structure and Lead Activation Mechanism on Cassiterite Surface
-
摘要:
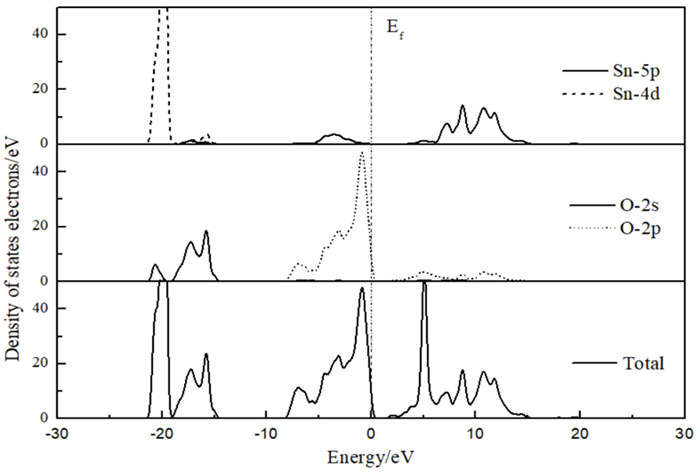
用密度泛函理论对锡石表面晶体结构及铅离子活化对苯甲羟肟酸和水杨羟肟酸在锡石表面的吸附过程的影响进行了研究。利用态密度及前线轨道理论分析了铅吸附后锡石(100)表面电子结构的变化。结果表明:铅离子吸附在锡石(100)后会降低表面氧原子的反应活性,增加表面的反应位点。根据前线轨道理论,从能量角度比较了苯甲羟肟酸和水杨羟肟酸与锡石(100)表面的相互作用能,揭示了羟肟酸浮选锡石的本质。
Abstract:The influence of the crystal structure of cassiterite surface and the activation of lead ion on the adsorption process of benzohydroxamic acid and salicylic oxime on cassiterite surface was studied by density functional theory. The electronic structure of cassiterite (100) after lead adsorption is analyzed by using the density of States and frontier orbital theory. The results show that the adsorption of lead ions on cassiterite (100) will reduce the reactivity of the surface oxygen atoms and increase the reaction sites on the surface. According to the frontier orbital theory, the interaction energy of Benzohydroxamic acid and salicylic hydroxamic acid and cassiterite (100) surface is compared from the energy point of view, and the essence of hydroxamic acid can be used in the flotation of cassiterite.
-

-
表 1 锡石不同解理面的表面能
Table 1. The surface energy of the different dissociation surfaces
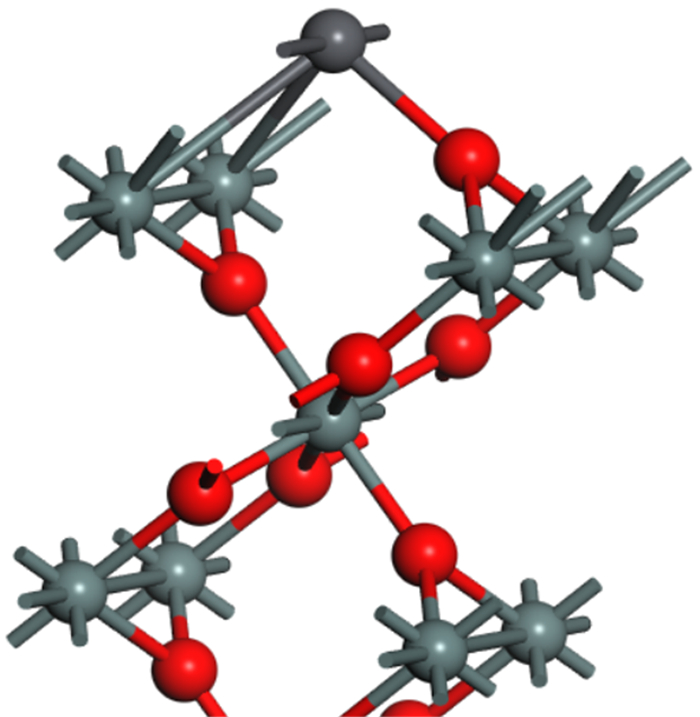
晶面指数 表面能/(J·m-2) 100 0.082 010 0.082 001 0.126 011 0.085 101 0.085 110 0.099 111 0.120 表 2 锡石晶体电子结构及铅离子对锡石(100)表面电子结构的影响
Table 2. The electronic structure of cassiterite crystals and the influence of lead ions on the electronic structure of cassiterite (100) surface
矿物 元素 s/e p/e d/e 电子数/e 电荷/e 锡石晶体 Sn 0.88 1.08 10 11.96 2.04 O 1.87 5.15 0 7.02 -1.02 锡石(100)面 Sn 0.99 1.05 10 12.04 1.96 O 1.93 5.01 0 6.94 -0.94 铅活化 Pb 1.81 1.55 9.99 13.35 0.65 Sn 1.47 1.31 10 12.77 1.23 O 1.93 4.97 0 6.90 -0.90 表 3 锡石铅活化前后和羟肟酸前线轨道能量分析
Table 3. Frontier orbital energy analysis before and after activation of cassiterite (100) surface
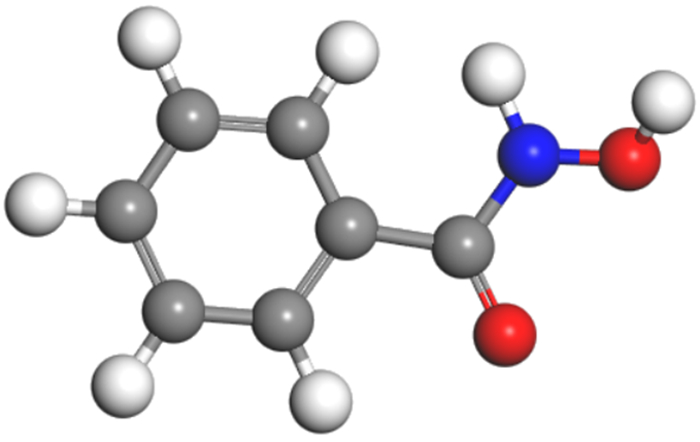
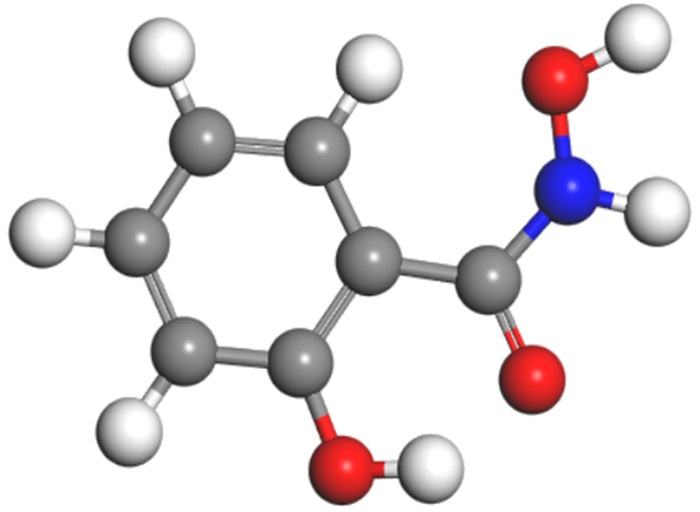
矿物/药剂 前线轨道 轨道能量/eV ΔE1/eV ΔE2/eV 锡石 HOMO -21.567 LUMO -21.507 铅活化 HOMO -21.133 LUMO -20.972 苯甲羟肟酸 HOMO -5.567 15.946 17.015 LUMO -4.552 15.411 16.581 水杨羟肟酸 HOMO -5.749 15.758 16.633 LUMO -4.934 15.223 16.199 ΔE1=|E(HOMO-reagent)-E(LUMO-mineral)|; ΔE2=|E(HOMO-mineral)-E(LUMO-reagent)|。 -
[1] 刘树仁.尚不为人们熟知的锡的用途[J].中国有色冶金, 1983(9):58-62. http://www.cnki.com.cn/Article/CJFDTotal-ZGDC200923012.htm
[2] 吴荣庆.我国锡矿综合利用水平有待提高[J].中国金属通报, 2009(9):32-33. http://www.cqvip.com/QK/84436A/201516/665655397.html
[3] 王晓, 童雄, 周永诚.锡石工艺矿物学与选矿工艺[J].矿冶, 2011, 20(4):15-19. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y2195839
[4] 百熙.浮选药剂[M].北京:冶金工业出版社, 1981.
[5] 王淀佐.浮选剂作用原理及应用[M].北京:冶金工业出版社, 1982.
[6] 王文潜.锡石浮选工艺研究[J].云南冶金, 1974(2):10-23. http://mall.cnki.net/magazine/Article/YSXK201403010.htm
[7] B. Jańczuk, M.L. González-Martín, Bruque J M. Wettability of cassiterite in presence of sodium dodecyl sulphate[J]. Materials Chemistry & Physics, 1994, 38(3):225-233. http://www.sciencedirect.com/science/article/pii/0254058494901961
[8] 罗建中.各类锡石捕收剂对细粒锡石和赤铁矿的浮选性能研究[J].有色金属(选矿部分), 1988(2):14-18. http://industry.wanfangdata.com.cn/yj/Detail/Thesis?id=Thesis_Y1718873
[9] B. Jańczuk, J.M. Bruque, M.L. González-Martin, et al. The contribution of double layers to the free energy of interactions in the cassiterite-SDS solution system[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 1995, 100(95):93-103. http://www.sciencedirect.com/science/article/pii/092777579503176E
[10] 王孝愈.苯乙烯膦酸浮选锡细泥工业试验[J].有色金属(选矿部分), 1980(3):44-46. http://www.cnki.com.cn/Article/CJFDTOTAL-YSXK198003016.htm
[11] Jones R O. The density functional formalism, its applications and prospects[J]. Reviews of Modern Physics, 1989, 61(3):689-746. doi: 10.1103/RevModPhys.61.689
[12] Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1998, 77(18):3865-3868. http://www.nrcresearchpress.com/servlet/linkout?suffix=refg28/ref28&dbid=16&doi=10.1139%2Fv11-044&key=10.1103%2FPhysRevLett.77.3865
[13] 孙伟, 柯丽芳, 孙磊.苯甲羟肟酸在锡石浮选中的应用及作用机理研究[J].中国矿业大学学报, 2013, 42(1):62-68. http://www.cnki.com.cn/Article/CJFDTotal-ZGKD201301013.htm
[14] Baur W H, Khan A A. Rutile-type compounds. Ⅳ. SiO2, GeO2 and a comparison with other rutile-type structures[J].Acta Crystallographica, 2010, 27(11):2133-2139. http://onlinelibrary.wiley.com/doi/10.1107/S0567740871005466/abstract
[15] Ren L, Qiu H, Zhang M, et al. Behavior of lead ions in cassiterite flotation using octanohydroxamic acid[J]. Industrial & Engineering Chemistry Research, 2017, 56(30):8723-8728.
[16] Fukui K, Koga N, Fujimoto H. Interaction frontier orbitals[J]. Cheminform, 1981, 103(1):196-197.
[17] Feng Q, Zhao W, Wen S, et al. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector[J]. Separation & Purification Technology, 2017, 178:193-199.
-




 下载:
下载: