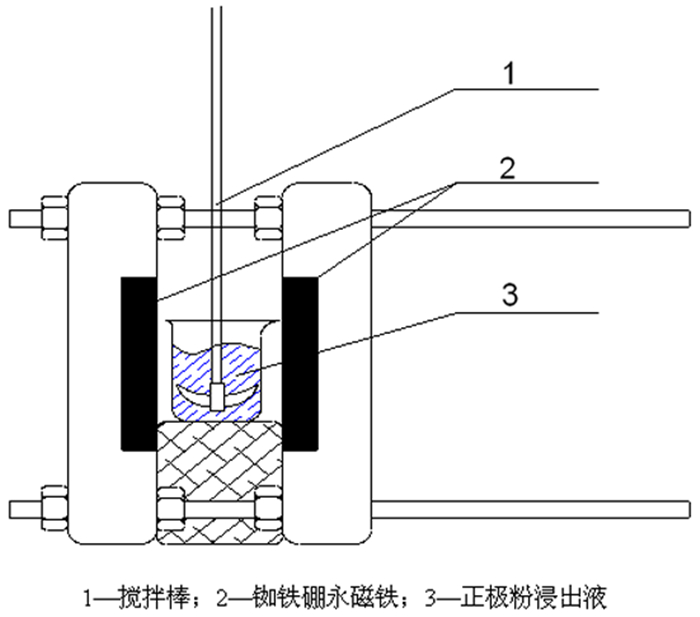
Study on Magnetic Field Intensification of Cobalt Leaching From Anode Powder of Used Lithium Battery
-
摘要:
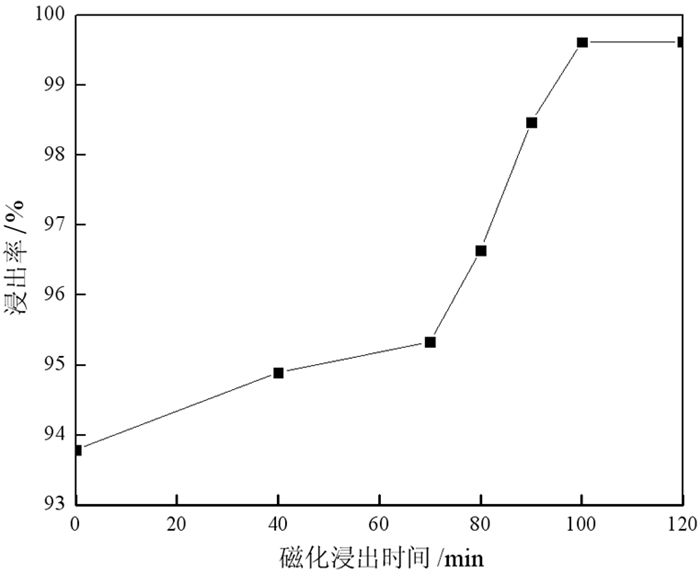
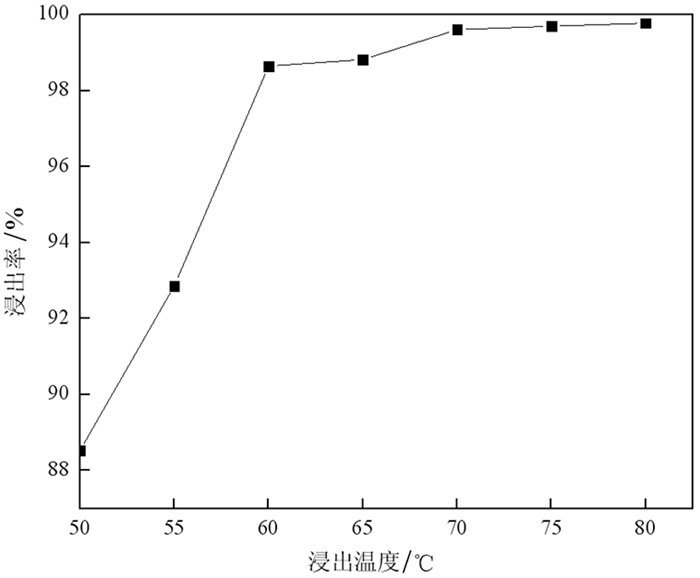
以废旧锂电池正极粉为原料,在磁场条件下,采用硫酸-双氧水体系浸出正极粉中的钴,探讨了磁感应强度、磁化浸出时间和浸出温度对钴浸出率的影响。结果表明,在磁感应强度为230 mT磁场、浸出时间为100 min、反应温度为70 ℃、固液比为1:100(其中硫酸浓度为3 mol/L)条件下,加入3 mL/g H2O2进行试验,钴的浸出率达到99.61%,相比较未磁化同等条件下,钴的浸出率提高了6.02个百分点。同时在硫酸用量减少20%的情况下,磁场强化浸出可以提高钴的浸出率4.62个百分点。磁场强化浸出的机理是加快了氢离子的扩散速度以及促进双氧水对Co3+的还原,从而提高了该浸出反应中钴的浸出率。
Abstract:Using used lithium battery cathode powder as raw material, under the condition of a magnetic field, the cobalt in the cathode powder is leached by sulfuric acid-hydrogen peroxide system. The influence of magnetic induction intensity, magnetization leaching time and leaching temperature on the cobalt leaching rate are discussed. The results show that, in a magnetic field of 230 mT, the leaching time is 100 min, the reaction temperature is 70 ℃, and the solid-to-liquid ratio is 1:100 (the sulfuric acid concentration is 3 mol/L), and 3 mL/g H2O2 is added for the test. The leaching rate of cobalt reaches 99.61%. Compared with the same condition without magnetization, the leaching rate of cobalt is increased by 6.02 percentage point. At the same time, when the amount of sulfuric acid is reduced by 20%, magnetic field enhanced leaching can increase the leaching rate of cobalt by 4.62 percentage point. The mechanism of magnetic field-enhanced leaching is to accelerate the diffusion rate of hydrogen ions and promote the reduction of Co3+ by hydrogen peroxide, thereby increasing the leaching rate of cobalt in the leaching reaction.
-
Key words:
- used lithium battery /
- positive electrode powder /
- magnetization /
- cobalt /
- leaching rate
-

-
表 1 正极粉中金属元素含量
Table 1. Metal element content in cathode powder
/% 成分 Li Co Ni Mn Al 含量 7.48 4.60 11.55 41.37 0.27 表 2 不同磁块间距下磁场中心位置的磁感应强度
Table 2. Magnetic induction intensity at the center of the magnetic field under different magnetic block spacing
磁块间距/cm 7 8 9 10 11 12 13 14 15 16 17 中心磁感应强度/mT 290 230 195 160 138 115 100 85 72 61 50 注:磁感应强度由SG-3M型特斯拉计测得。 表 3 磁场强化硫酸浸出与常规硫酸浸出对比
Table 3. Comparison of magnetic field enhanced sulfuric acid leaching and conventional sulfuric acid leaching
浸出方式 硫酸浓度/(mol·L-1) 磁感应强度/mT 双氧水用量/(mL·g-1) 温度/℃ 固液比 磁化时间/min 浸出率/% 常规浸出 3 0 3 70 1:100 0 93.59 磁化浸出 3 230 3 70 1:100 100 99.61 磁化浸出 3 230 3 70 1:90 90 98.39 磁化浸出 3 230 3 70 1:80 100 98.21 -
[1] GU F, GUO JF, YAO X, et al. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China[J]. Journal of Cleaner Production, 2017, 161: 765-780. doi: 10.1016/j.jclepro.2017.05.181
[2] PEREZ E, ANDRE M L, AMADOR R N, et al. Recovery of metals from simulant spent lithium-ion battery as organophosphonate coordination polymers in aqueous media[J]. Journal of Hazardous Materials, 2016, 317: 617-621. doi: 10.1016/j.jhazmat.2016.06.032
[3] 郝涛, 张英杰, 董鹏, 等. 废旧三元动力锂离子电池正极材料回收的研究进展[J]. 硅酸盐通报, 2018, 37(8): 2450-2456. https://www.cnki.com.cn/Article/CJFDTOTAL-GSYT201808018.htm
[4] LV W G, WANG Z H, CAO H B, et al. A critical review and analysis on the recycling of spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1504-1521. http://pubs.acs.org/doi/abs/10.1021/acssuschemeng.7b03811
[5] WEI S P, SUN J, ZHOU T, et al. Research development of metals recovery from spent lithium-ion batteries[J]. Energy Storage Science and Technology, 2017, 6(6): 1196-1207. http://en.cnki.com.cn/Article_en/CJFDTotal-CNKX201706004.htm
[6] 张英杰, 宁培超, 杨轩, 等. 废旧三元锂离子电池回收技术研究新进展[J]. 化工进展, 2020, 39(7): 2828-2840. https://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ202007037.htm
[7] 谢英豪, 欧彦楠, 余海军, 等. 废旧车用动力电池安全放电研究[J]. 工业安全与环保, 2017, 43(9): 44-47. doi: 10.3969/j.issn.1001-425X.2017.09.013
[8] Zhang P W, Yokoyama T, Itabashi O, et al. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. 1998, 47(2): 259-271. Yusuf K O, Ogunlela A O. Impact of magnetic treatment of irrigation water on the growth and yield of tomato[J]. Notulae Scientia Biologicae, 2015, 7(3): 345-348. doi: 10.15835/nsb739532
[9] 陈亮, 唐新村, 张阳, 等. 从废旧锂离子电池中分离回收钴镍锰[J]. 中国有色金属学报, 2011, 21(5): 1192-1198. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201105039.htm
[10] Yusuf K O, Ogunlela A O. Impact of magnetic treatment of irrigation water on the growth and yield of tomato[J]. Notulae Scientia Biologicae, 2015, 7(3): 345-348. doi: 10.15835/nsb739532
[11] GERALDO B N, RODINI E F J, QUIRINO L M J, et al. Water treatment by magnetic field increases bone mineral density of rats[J]. Journal of Clinical Densitometry, 2017, 20(4): 526-531. doi: 10.1016/j.jocd.2017.06.002
[12] 夏青, 王健. 提高含铜难浸金矿金浸出率的细菌预处理研究[J]. 金属矿山, 2010(5): 77-80. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201005023.htm
[13] 卢丽丽, 廖婵娟, 邓娜, 等. 磁场对细菌浸出尾矿中铜、锌的影响[J]. 当代化工, 2015, 44(11): 2524-2527. doi: 10.3969/j.issn.1671-0460.2015.11.005
[14] 牛梓璇, 胡源, 张艳, 等. 磁效应对水处理的影响研究[J]. 广州化工, 2016, 44(16): 21-25. doi: 10.3969/j.issn.1001-9677.2016.16.006
[15] 程海翔, 张辉, 徐天有, 等. 铜矿尾矿资源化利用研究进展[J]. 化工进展, 2015, 34: 192-195. https://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2015S1036.htm
[16] 卢丽丽. 电磁场强化细菌浸出铜尾矿重金属技术研究[D]. 重庆: 重庆大学, 2012.
-



 下载:
下载: