Hydrothermal Transformation of Natural Clinoptilolite Zeolite to Zeolite Na-P and Its Adsorption Property for Cd ion
-
摘要:
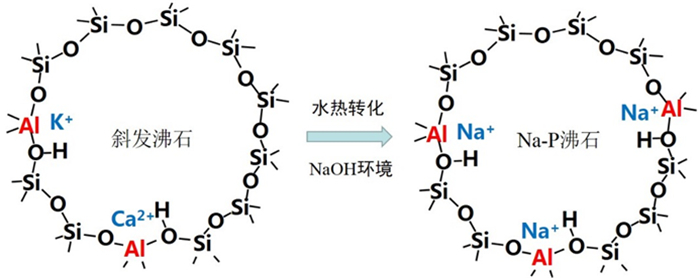
以河北承德围场地区天然沸石为原料,对其进行水热改性制备Na-P沸石。采用X射线衍射仪、X射线光电子能谱仪、气体吸附仪、扫描电镜和能谱分析仪等对材料进行表征分析,采用Cd2+离子评价材料的重金属离子去除性能。研究碱处理浓度、水热温度和时间对天然沸石结构的影响规律,着重研究所制备Na-P沸石的组成结构特征及重金属离子去除性能。结果表明:在NaOH浓度3 mol/L、水热温度100 ℃、水热时间12 h的条件下,斜发沸石可转化为纯度较高的Na-P沸石。所得材料对Cd2+的吸附容量为35.7 mg/g,较天然沸石提升7倍。Na-P沸石性能的提升主要归功于其较低的硅铝比和较高的表面Na+含量。
Abstract:Natural clinoptilolite zeolites from Weichang region, Chengde city, Hebei province were transformed to a zeolite Na-P using hydrothermal method. The as-prepared materials were characterized and analyzed by X-ray diffraction, X-ray photoelectron spectroscopy, gas adsorption instrument, scanning electron microscope and energy spectrum analyzer. The heavy metal ions removal performance was evaluated by Cd2+ ion. The effects of alkali treatment concentration, hydrothermal temperature and time on the structure of natural zeolite were investigated. The composition, structural characteristics and heavy metal ion removal performance of the prepared zeolite Na-P materials were further studied. The results showed that clinoptilolite could be transform to zeolite Na-P with 3 mol/L NaOH solution under the conditions of hydrothermal temperature of 100 ℃ and hydrothermal time of 12 h. The adsorption capacity of the obtained material for Cd2+ was 35.7 mg/g, which was 7 times higher than that of natural zeolite. Its excellent performance was mainly due to low silicon/aluminum ratio and relative higher exchangeable Na ion content.
-
Key words:
- natural clinoptilolite /
- hydrothermal treatment /
- zeolite Na-P /
- Cd2+
-

-
[1] MISAELIDES P. Application of natural zeolites in environmental remediation: A short review[J]. Microporous and Mesoporous Materials, 2011, 144: 15-18. doi: 10.1016/j.micromeso.2011.03.024
[2] 李超, 王丽萍. 矿物材料处理废水的研究进展[J]. 矿产保护与利用, 2020, 40(1): 65-71. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=a3b2a301-2fca-4fbd-a0eb-b8ce86b6bf2a
[3] 佘振宝. 沸石加工与应用: 第2版[M]. 北京: 化学工业出版社, 2013.
[4] 杨磊. 改型斜发沸石与钙、镁离子水溶液体系离子交换平衡研究[D]. 天津: 河北工业大学, 2010.
[5] YANG S J, LACH-HAB M, VAISMAN I I, et al. Framework-type determination for zeolite structures in the inorganic crystal structure database[J]. Journal of Physical and Chemical Reference Data, 2010, 39: 1-45.
[6] BEHIN J., KAZEMIAN H., ROHANI S. Sonochemical synthesis of zeolite NaP from clinoptilolite[J]. Ultrasonics Sonochemistry, 2016, 28: 400-408. doi: 10.1016/j.ultsonch.2015.08.021
[7] ALDAHRI T, BEHIN J, KAZEMIAN, et al. Synthesis of zeolite Na-P from coal fly ash by thermo-sonochemical treatment[J]. Fuel, 2016, 182: 494-501. doi: 10.1016/j.fuel.2016.06.019
[8] LIU Y, YAN C J, ZHAO J J, et al. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water[J]. Journal of Cleaner Production, 2018, 202: 11-22. doi: 10.1016/j.jclepro.2018.08.128
[9] ALIAS M Y, NIK A N N M, NURUL A K, et al. Removal of Ca2+ and Zn2+ from aqueous solutions by zeolites NaP and KP[J]. Environmental Technology, 2010, 31: 41-46. doi: 10.1080/09593330903313794
[10] ZHANG Y N, CHEN Y G, KANG W, et al. Excellent adsorption of Zn (Ⅱ) using NaP zeolite adsorbent synthesized from coal fly ash via stage treatment[J]. Journal of Cleaner Production, 2020, 258: 120736. doi: 10.1016/j.jclepro.2020.120736
[11] NERY J G, YVONNE P M, ANTHONY K C. A study of the highly crystalline, low-silica, fully hydrated zeolite P ion exchanged with (Mn2+, Cd2+, Pb2+, Sr2+, Ba2+) cations[J]. Microporous and Mesoporous Materials, 2003, 57: 229-248. doi: 10.1016/S1387-1811(02)00594-2
[12] CHENM Y, NONG S Y, ZHAO Y T, et al. Renewable P-type zeolite for superior absorption of heavy metals: Isotherms, kinetics, and mechanism[J]. Science of the Total Environment, 2020, 726: 138535. doi: 10.1016/j.scitotenv.2020.138535
[13] LIU Y, WANG G D, WANG L, et al. Zeolite P synthesis based on fly ash and its removal of Cu (Ⅱ) and Ni (Ⅱ) ions[J]. Chinese Journal of Chemical Engineering, 2019, 27: 341-348. doi: 10.1016/j.cjche.2018.03.032
[14] DENG H, GE Y. Formation of NaP zeolite from fused fly ash for the removal of Cu(Ⅱ) by an improved hydrothermal method[J]. RSC Advances, 2015, 5: 9180-9188. doi: 10.1039/C4RA15196H
[15] KANG S J, KAZUHIKO E, AKIRA Y. Transformation of a low-grade Korean natural zeolite to high catadsorbent by hydrothermal reaction with or without fusion with sodium hydroxide[J]. Applied Clay Science, 1998, 13: 117-135. doi: 10.1016/S0169-1317(98)00019-2
[16] WANG Y F, FENG L. Synthesis of high capacity catadsorbents from a low-grade Chinese natural zeolite[J]. Journal of Hazardous Materials, 2009, 166: 1014-1019. doi: 10.1016/j.jhazmat.2008.12.001
[17] LIMLAMTHONG M, LEE M, JONGSOMJIT B, et al. Solution-mediated transformation of natural zeolite to ANA and CAN topological structures with altered active sites for ethanol conversion[J]. 2021, 32: 4155-4166.
[18] WANG C, LENG S Z, GUO H D, et al. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite[J]. Applied Surface Science, 2019, 478: 319-326. doi: 10.1016/j.apsusc.2019.01.263
[19] WANG X Y, PLACKOWSKI C X, NGUYEN A V X-ray photoelectron spectroscopic investigation into the surface effects of sulphuric acid treated natural zeolite[J]. Powder Technology, 295: 27-34.
[20] CORMA A, FORNES V, PALLOTA O, et al. Determination of framework and non-framework aluminium in HY dealuminated zeolites by X-ray photoelectron spectroscopy[J]. Journal of the Chemical Society, Chemical Communications, 1986, 333-334.
-




 下载:
下载: