Flotation Properities of Rutile Using Salts of Benzohydroxamic and Octadecenoic (Octadecanoic) Acids as Synergistic Collectors
-
摘要:
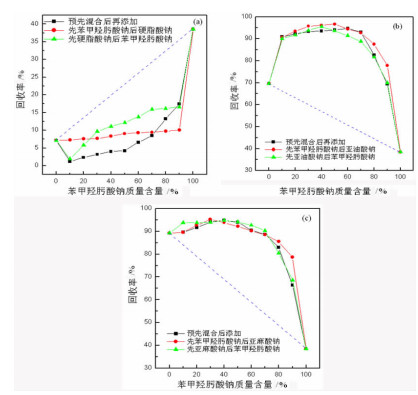
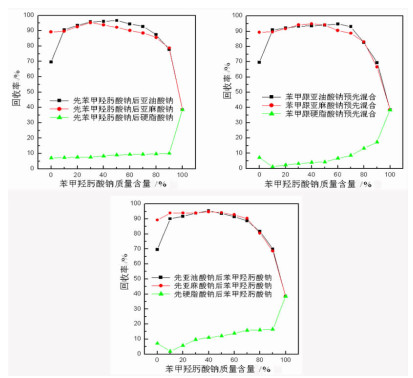
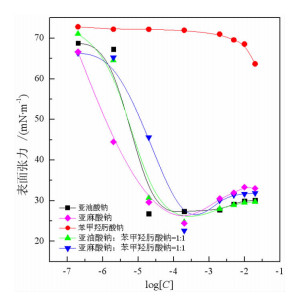
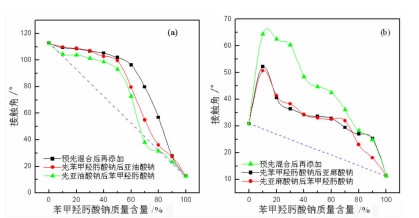
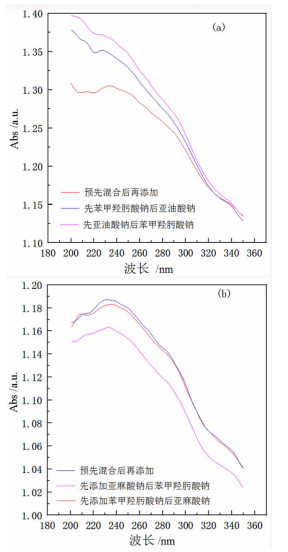
研究了金红石在苯甲羟肟酸钠与C18烷(烯)酸钠复合体系中的浮选特性, 采用多种界面化学检测手段研究了组合药剂之间以及组合药剂与金红石的作用机理。结果表明: 试验条件下四种药剂对金红石捕收能力的大小顺序为: 亚麻酸钠(εmax=89.23%, ε为回收率)>亚油酸钠(εmax=69.57%)>苯甲羟肟酸钠(εmax=38.43%)>硬脂酸钠(εmax=7.13%)。具有相同碳原子数的脂肪酸钠分子中的双键数目对药剂的捕收能力及药剂组合的协同效应影响较大, 深层次的原因则是双键数目的多少可对脂肪酸类药剂的krafft点、分子结构及其与苯甲羟肟酸钠之间的作用力产生不同影响。三种药剂组合对金红石捕收能力的大小顺序为: 苯甲羟肟酸钠+亚油酸钠≈苯甲羟肟酸钠+亚麻酸钠≫苯甲羟肟酸钠与硬脂酸钠。脂肪酸类药剂分子中的双键可与苯甲羟肟酸钠分子中的苯环产生电子共轭效应, 该效应所生成的缔合物种类及其空间构象是影响组合药剂浮选效果的关键因素。苯甲羟肟酸钠和亚油酸钠体系中金红石的浮选行为与其带隙变化有较好的对应关系, 但苯甲羟肟酸钠与亚麻酸钠体系中的对应关系则不明显, 具体原因则有待于进一步研究。
Abstract:Flotation responses of rutile in various systems of sodium benzohydroxamide (BHA) combined with several fatty acid salts collectors were studied and the interaction mechanisms of the reagents, as well as that of the reagents onto the mineral have been studied using various measurements, namely, Zeta potential, surface tension, contact angle and UV diffuse reflectance spectroscope. The results indicate that the collecting ability (recovery) of the single reagent follows the order: sodium linolenate (89.23%) > sodium linoleate (69.57%) > sodium benzohydroxamide (38.43%) ≫ sodium stearate (7.13%). The number of the double bond in sodium salts of fatty acids bearing the same length of hydrocarbon chain has a significant influence on the reagent collecting ability and the synergistic effect of reagent combination, however, the deep reason is that the double bond has an important impact on such properties as Krafft point, molecular structure of fatty-acid collector as well as the interactions of fatty-acid collectors with BHA. The collecting ability of the three reagent combinations is sodium benzohydroxamide + sodium linoleate(SL) ≈ sodium benzohydroxamide + sodium linolenate (SN) ≫ sodium benzohydroxamide + sodium stearate (ST). It is observed that there is an electron conjugation effect among the double bonds in fatty-acid collector and the benzene ring in BHA which is the main reason for the formation of the association complex between the reagents. The amount and the configuration of the complexes could be critical for the collecting performance of reagent combination.
-
Key words:
- rutile /
- flotation /
- collector /
- synergistic reagents /
- interfacial organization
-

-
表 1 本研究所用主要仪器及药品
Table 1. The main instruments and chemicals used in this study
名称 型号 生产厂家 纯度 挂槽浮选机 RK/FGC 武汉洛克粉磨设备制造有限公司 - 电泳仪 JS94H 上海中晨数字技术设备有限公司 - 接触角仪 JY-82 承德鼎盛试验机检测设备有限公司 - 自动表面张力仪 BZY-1 上海恒平仪器有限公司 - 紫外-可见-近红外分光光度计 Agilent5000 美国安捷伦科技公司 - 亚油酸钠 Macklin 上海麦克林生化科技有限公司 99% 亚麻酸钠 aladdin 阿拉丁试剂(上海)有限公司 99% 苯甲羟肟酸钠 Macklin 上海麦克林生化科技有限公司 99% NaOH 天力 天津天力化学试剂有限公司 化学纯 盐酸 坤丰 济南坤丰化工有限公司 化学纯 水 / 自制 超纯水 表 2 不同药剂组合的协同效应
Table 2. Synergism of different reagent combinations
苯甲羟肟酸钠质量含量/% 协同效应指数 先加入苯甲羟肟酸钠 预先混合 后加入苯甲羟肟酸钠 后加入亚油酸钠 后加入亚麻酸钠 与亚油酸钠混合 与亚麻酸钠混合 先加入亚油酸钠 先加亚麻酸钠 0 0.00 0.00 0.00 0.00 0.00 0.00 10 0.36 0.06 0.37 0.06 0.36 0.11 20 0.47 0.17 0.45 0.15 0.45 0.18 30 0.59 0.28 0.55 0.27 0.56 0.27 40 0.68 0.36 0.64 0.37 0.67 0.37 50 0.79 0.44 0.74 0.47 0.73 0.47 60 0.85 0.53 0.86 0.53 0.80 0.57 70 0.94 0.65 0.94 0.65 0.86 0.68 80 0.96 0.76 0.85 0.70 0.83 0.65 90 0.87 0.81 0.67 0.52 0.68 0.57 100 0.00 0.00 0.00 0.00 0.00 0.00 表 3 不同体系中浮选指标的拟合曲线
Table 3. Fitting equations of flotation index for different reagent combination systems
药剂制度 拟合曲线 R2 SD P值 预先混合后再添加 苯甲羟肟酸钠+亚油酸钠 ε=71.00+2.34n-0.08n2+10-3n3-6.74×10-6n4 0.99 2.26 < 10-4 苯甲羟肟酸钠+亚麻酸钠 ε=90.38-0.27n+0.017n2-2.00×10-4n3 0.98 2.65 < 10-4 先添加苯甲羟肟酸钠 后添加亚油酸钠 ε=69.83+2.82n-0.10n2+1.50×10-3n3-8.58×10-6n4 0.99 2.51 < 10-4 后添加亚麻酸钠 ε=89.51-0.75n+0.10n2-3.00×10-3n3+4.25×10-4n4-1.94×10-7n5 1.00 1.52 < 10-4 后添加苯甲羟肟酸钠 先添加亚油酸钠 ε=70.33+2.44n-0.09n2+10-3n3-6.56×10-6n4 0.99 1.96 < 10-4 先添加亚麻酸钠 ε=89.49+0.50n-0.016n2+1.78×10-4n3+4.79×10-7n4-1.64×10-8n5 1.00 1.22 < 10-4 注:ε表示100×(金红石回收率);n表示100×(苯甲羟肟酸钠质量含量)。 -
[1] 吴贤, 张健, 康新婷, 等. 我国金红石矿资源分布开发及技术现状[J]. 稀有金属, 2007, 31(S1): 146-150. https://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS2007S1034.htm
WU X, ZHANG J, KANG X T, et al. Distribution, exploiture and actualities of technique of rutile mineral resources in China[J]. Chinese Journal of Rare Metals, 2007, 31(S1): 146-150. https://www.cnki.com.cn/Article/CJFDTOTAL-ZXJS2007S1034.htm
[2] CAO M, GAO Y D, BU H, et al. Study on the mechanism and application of rutile flotation with benzohydroxamic acid[J]. Mineral Engineering, 2019(2): 275-280.
[3] 崇霄霄, 栾文楼, 王丰翔, 等. 全球钛资源现状概述及我国钛消费趋势[J]. 矿产保护与利用, 2020, 40(2): 162-170. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=71faf3be-58fa-4c19-92f3-3d7e66fa91fa
CHONG X X, LUAN W L, WANG F X, et al. Overview of global titanium resources status and titanium consumption trend in China[J]. Conservation and Utilization of Mineral Resources, 2020, 40(2): 162-170. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=71faf3be-58fa-4c19-92f3-3d7e66fa91fa
[4] 岳铁兵, 魏德州, 曹进成, 等. 细粒金红石矿浮选工艺研究[J]. 化工矿物与加工, 2005(3): 1-3. doi: 10.3969/j.issn.1008-7524.2005.03.001
YUE T B, WEI D Z, CAO J C, et al. Study on the flotation process of fine rutile ore[J]. IM & P, 2005(3): 1-3. doi: 10.3969/j.issn.1008-7524.2005.03.001
[5] PATRA A S, BASU A, BISWAS A, et al. Synergistic effect of polar group containing collectors and synthesized frother on flotation performance of oxidized coal[J]. International Journal of Coal Preparation and Utilization, 2021, 41(5): 1-14.
[6] 朱建光, 周菁. 钛铁矿、金红石和稀土选矿技术[M]. 长沙: 中南大学出版社, 2009.
ZHU J G, ZHOU J. Beneficiation technique for ilmenite, rutile and rare earth[M]. Changsha: Central South University Press, 2009.
[7] 刘明宝, 鱼博, 印万忠. 矿浆pH值对含苯环螯合捕收剂在金红石表面吸附速率的影响[J]. 过程工程学报, 2018, 18(2): 399-404. https://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201802026.htm
LIU M B, YU B, YIN W Z. Effect of Slurry pH on Adsorption Rate of Chelating Collectors Containing Benzene Ring onto Rutile Surface (in Chinese). Chin. J. Process Eng., 2018, 18(2): 399-404. https://www.cnki.com.cn/Article/CJFDTOTAL-HGYJ201802026.htm
[8] YANG S Y, XU Y L, LIU C, et al. Investigations on the synergistic effect of combined NaOl/SPA collector in ilmenite flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021: 127267.
[9] 徐龙华, 田佳, 巫侯琴, 等. 组合捕收剂在矿物表面的协同效应及其浮选应用综述[J]. 矿产保护与利用, 2017(2): 107-112. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=7068e474-71c6-4dcc-b4c0-0534a95e29a7
XU L H, TIAN J, WU H Q, et al. A Review on the Synergetic Effect of the Mixed Collectors on Mineral Surface and Its Application in Flotation[J]. Conservation and Utilization of Mineral Resources, 2017(2): 107-112. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=7068e474-71c6-4dcc-b4c0-0534a95e29a7
[10] 唐云, 杨典奇, 王雪, 等. 羟肟酸协同脂肪酸分离磷灰石和白云石[J]. 金属矿山, 2016(4): 86-90. doi: 10.3969/j.issn.1001-1250.2016.04.017
TANG Y, YANG D Q, WANG X, et al. Synergistic Effect of fatty acid and hydroxamic acid in flotation separation of apatite and dolomite[J]. Metal Mine, 2016(4): 86-90. doi: 10.3969/j.issn.1001-1250.2016.04.017
[11] WEI Q, DONG L, JIAO F, et al. The synergistic depression of lime and sodium humate on the flotation separation of sphalerite from pyrite[J]. Minerals Engineering, 2021, 163: 106779. doi: 10.1016/j.mineng.2021.106779
[12] 张闿. 浮选药剂的组合使用[M]. 北京: 冶金工业出版社, 1994: 58-61.
ZHANG K. Composed utilization of flotation reagent[M]. Beijing: Metallurgical Industry Press, 1994: 58-61.
[13] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 113.
WANG D Z, Hu Y H. Solution chemistry in flotation[M]. Changsha: Hunan Science and Technology Press, 1988: 113.
[14] TIAN Z R, VOIGT J A, LIU J, et al. Complex and oriented ZnO nanostructures[J]. Nat. Mater., 2003, 2: 821-826. doi: 10.1038/nmat1014
-



 下载:
下载: