Application and Quantum Chemical Analysis of Novel Sulfur-Containing Heterocyclic Inhibitors in Separation of Molybdenite and Galena
-
摘要:
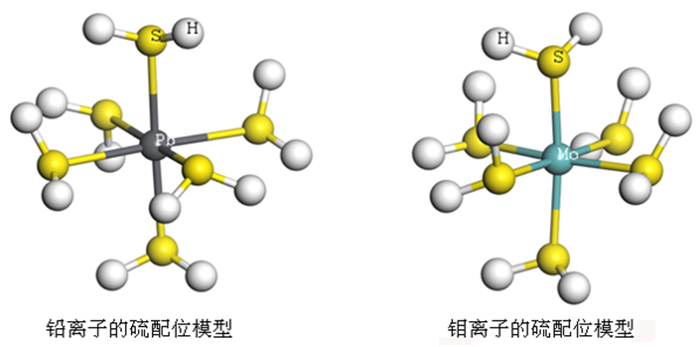
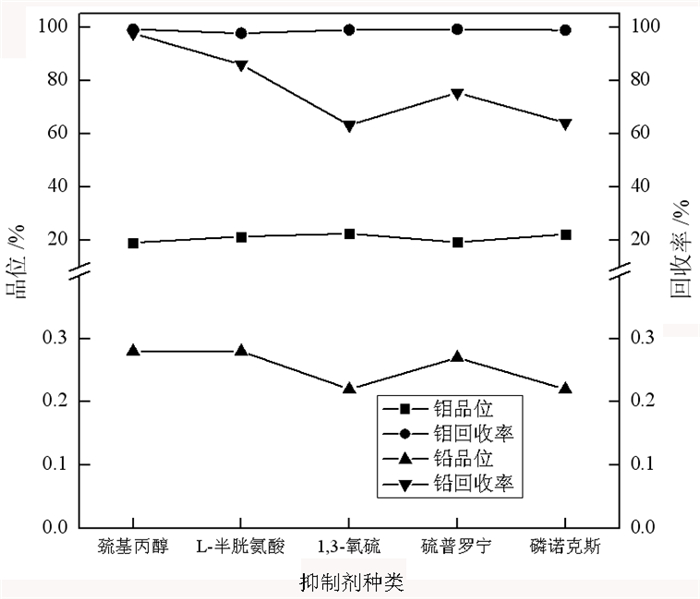
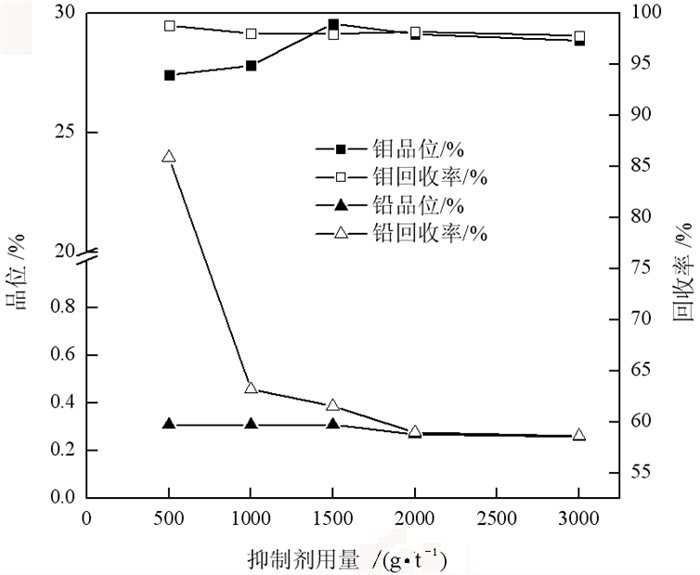
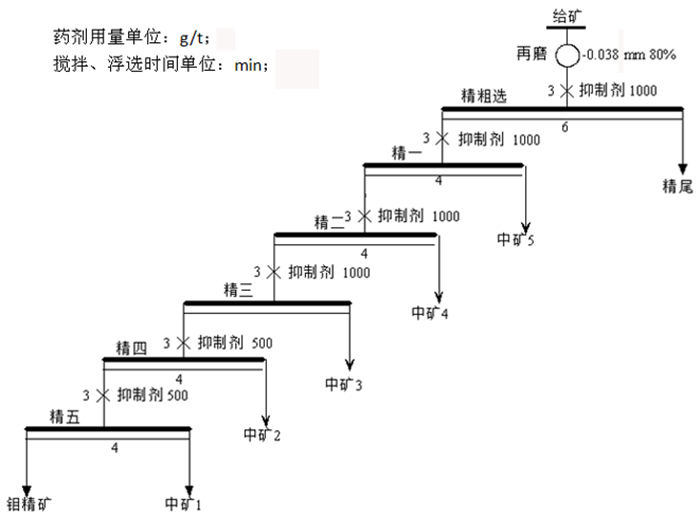
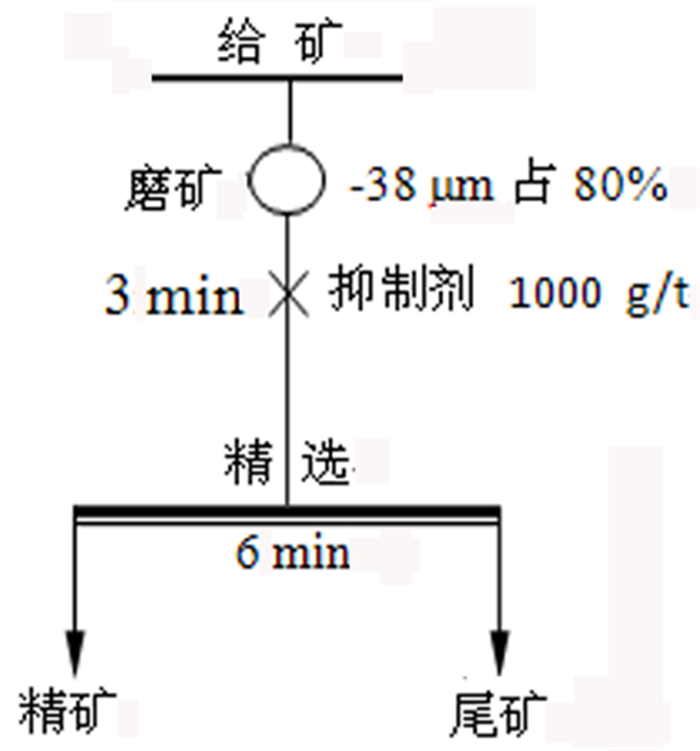
传统钼铅分离过程中使用磷诺克斯作为抑制剂, 存在毒性大、污染严重等问题。为了降低金堆城钼精矿中铅的含量, 对比了毒性较弱的巯基丙醇、L-半胱氨酸、1, 3-氧硫杂戊环-羧酸、硫普罗宁的铅抑制效果, 并对抑制剂用量进行了考察, 经过比较选取1, 3-氧硫杂戊环-羧酸作为金堆城钼铅混合精矿的抑制剂。经过条件试验, 最终确定在再磨细度-38 μm占80%时, 经过一次粗选、五次精选, 可以获得钼品位52.20%、回收率85.01%、含铅0.010%的钼精矿产品, 其效果与磷诺克斯相当。采用配位化学以及密度泛函理论计算了方铅矿和辉钼矿的前线轨道特征, 并通过对方铅矿和辉钼矿中金属位点的前线轨道特征进行分析指出, 钼铅分离抑制剂的前线轨道对称性是影响抑制剂选择性的关键。
Abstract:In the traditional separation of molybdenum and lead, phosphorus nox is used as an inhibitor, which has serious toxicity and pollution. In order to reduce the content of lead in molybdenum concentrate from Jinduicheng, the lead inhibition effects of mercaptopropanol, L-cysteine, 1, 3-oxythiopentane carboxylic acid and tiopronin with weak toxicity were compared, and the amount of inhibitor were investigated. After comparison, 1, 3-oxythiopentane carboxylic acid was selected as the inhibitor of molybdenum-lead-bearing concentrate in Jinduicheng. After condition test, molybdenum concentrate can be obtained with molybdenum grade of 52.20%, recovery rate of 85.01% and lead content of 0.010% through one roughings and five cleanings when the regrinding fineness of -38 μm was 80%. The effect of 1, 3-oxythiopentane carboxylic acid is equivalent to that of phosphorus Knox. The characteristics of frontier orbits of galena and molybdenite were calculated by means of coordination chemistry and density functional theory, and the characteristics of frontier orbits of metal sites in galena and molybdenite were analyzed. It is pointed out that the symmetry of frontier orbits of the inhibitors is the key to affect the selectivity of inhibitors.
-

-
表 1 原料多元素分析结果
Table 1. Multi-composition analysis result of raw ores
/% 元素 Mo Fe Pb Zn Cu K2O Na2O 含量 16.61 8 0.21 0.23 1.04 2.39 0.38 元素 P S CaO Ti MgO SiO2 Al2O3 含量 0.052 18.21 2.32 0.24 1.24 40.25 7.97 表 2 矿石的矿物组成及相对含量
Table 2. Mineral composition and relative content of ore
/% 矿物名称 含量 矿物名称 含量 辉钼矿 27.69 石英 33.19 黄铁矿 17.18 钾长石 13.58 闪锌矿 0.34 钠长石 3.22 黄铜矿 2.98 磷灰石 0.28 方铅矿 0.24 其他 0.89 金红石 0.41 表 3 分子结构示意图
Table 3. Molecular structures of depressant reagents
抑制剂 分子结构 巯基丙醇 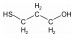
L-半胱氨酸 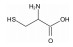
1, 3-氧硫杂戊环-羧酸 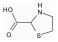
硫普罗宁 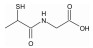
表 4 钼精矿降铅工艺开路试验结果
Table 4. Results of open circuit for lead reduction in molybdenum concentrate
/% 抑制剂种类 产品名称 作业产率 品位 作业回收率 Cu Mo Pb SiO2 Cu Mo Pb SiO2 1, 3-氧硫杂戊环-羧酸 钼精矿 24.07 0.057 52.20 0.010 5.74 1.03 85.01 1.10 3.65 中矿1 1.60 0.27 29.26 0.33 30.12 0.33 3.16 2.43 1.27 中矿2 5.40 0.69 5.68 0.38 58.52 2.80 2.08 9.42 8.34 中矿3 3.08 2.54 15.90 0.75 38.31 5.91 3.31 10.46 3.11 中矿4 5.87 4.89 5.96 0.82 41.03 21.72 2.37 21.77 6.35 中矿5 20.92 2.10 1.21 0.30 54.09 33.25 1.71 28.31 29.84 精尾 39.07 1.18 0.89 0.15 46.03 34.95 2.36 26.52 47.44 给矿 100.00 1.32 14.78 0.22 37.91 100.00 100.00 100.00 100.00 磷诺克斯 钼精矿 24.41 0.044 51.92 0.010 5.34 0.84 85.20 1.08 3.41 中矿1 1.13 0.21 23.96 0.069 31.81 0.19 1.82 0.35 0.94 中矿2 5.62 0.41 7.58 0.11 52.30 1.78 2.86 2.73 7.69 中矿3 3.39 1.49 19.23 0.25 36.75 3.89 4.38 3.81 3.26 中矿4 4.56 3.83 4.14 0.61 44.93 13.51 1.27 12.40 5.36 中矿5 20.57 2.07 1.46 0.37 54.38 32.87 2.01 33.32 29.28 精尾 40.33 1.51 0.91 0.26 47.43 46.92 2.45 46.32 50.06 给矿 100.00 1.29 14.87 0.23 38.21 100.00 100.00 100.00 100.00 表 5 前线轨道分析
Table 5. The analysis of frontier molecular orbitals
/eV 前线轨道能 巯基丙醇 L-半胱氨酸 1, 3-氧硫杂戊环-羧酸 硫普罗宁 方铅矿 辉钼矿 E(HOMO) -5.215 -5.540 -5.479 -6.044 -11.464 -22.612 E(LUMO) -1.077 -2.331 -2.113 -2.745 -9.253 -22.146 ΔE1 4.038 3.68 3.774 3.656 ΔE2 10.387 9.933 9.351 9.724 ΔE3 16.931 16.573 16.667 16.549 ΔE4 21.535 21.081 20.499 20.872 注:ΔE1=|E(HOMO-药剂)-E(LUMO-方铅矿)|; ΔE2=|E(HOMO-方铅矿)-E(LUMO-药剂)|;ΔE3=|E(HOMO-药剂)-E(LUMO-辉钼矿)|;ΔE4=|E(HOMO-辉钼矿)-E(LUMO-药剂)|。 -
[1] 贾红秀, 高丽梅, 姜威. 钼市场30年回顾与展望[J]. 中国钼业, 2006(1): 42-47. doi: 10.3969/j.issn.1006-2602.2006.01.012
JIA H X, GAO L M, JIANG W. Review of the 30 year s' molybdenum market and its prospect[J]. China Molybdenum Industry, 2006(1): 42-47. doi: 10.3969/j.issn.1006-2602.2006.01.012
[2] 杜科让. 我国钼矿资源开采利用现状及存在的问题分析[J]. 科技资讯, 2011(19): 148. doi: 10.3969/j.issn.1672-3791.2011.19.109
DU K R. Analysis on the present situation and existing problems of molybdenum mining and utilization in China[J]. Science & Technology Information, 2011(19): 148. doi: 10.3969/j.issn.1672-3791.2011.19.109
[3] 张文钲. 钼精矿降铅方法[J]. 中国钼业, 2003(4): 5-6+17. doi: 10.3969/j.issn.1006-2602.2003.04.002
ZHANG W Z. Method of reducing lead from molybdenite concentrates[J]. China Molybdenum Industry, 2003(4): 5-6+17. doi: 10.3969/j.issn.1006-2602.2003.04.002
[4] 李永贵. 磷诺克斯中毒及预防[J]. 中国钼业, 1999, 23(2): 35-38. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMY902.011.htm
LI Y G. The poisoning of pnokes and it' s prevention[J]. China Molybdenum Industry, 1999, 23(2): 35-38. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMY902.011.htm
[5] 陈建华, 冯其明. 新型有机抑制剂CTP对硫化矿的抑制性能[J]. 湖南有色金属, 1995(6): 29-30. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYJ506.009.htm
CHEN J H, FENG Q M. Inhibition performance of new organic inhibitor CTP on sulfide ore[J]. Hunan Nonferrous Metals, 1995(6): 29-30. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYJ506.009.htm
[6] 刘润清, 孙伟, 胡岳华. 铜铅分离有机抑制剂FCLS的研究[J]. 矿冶工程, 2009, 29(3): 29-32. doi: 10.3969/j.issn.0253-6099.2009.03.009
LIU R Q, SUN W, HU Y H. Study on organic depressant FCLS for separation of chalcopyrite and galena[J]. Mining And Metallurgical Engineering, 2009, 29(3): 29-32. doi: 10.3969/j.issn.0253-6099.2009.03.009
[7] 岳紫龙, 成建, 刘威. 河南某钼铅硫矿选矿试验研究[J]. 有色金属(选矿部分), 2015(1): 21-25. doi: 10.3969/j.issn.1671-9492.2015.01.006
YUE Z L, CHENG J, LIU W. The mineral processing research on a molybdenum-lead-sulfur ore in henan[J]. Nonferrous Metals(Mineral Processing Section), 2015(1): 21-25. doi: 10.3969/j.issn.1671-9492.2015.01.006
[8] 武俊杰, 孙阳, 缑明亮, 等. 陕西某钼铅多金属矿选矿试验[J]. 金属矿山, 2014, 32(11): 75-79. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201411018.htm
[9] WU J J, SUN Y, GOU M L, et al. Beneficiation test of Mo-Pb polymetallic ore in shaanxi[J]. Metal Mine, 2014, 32(11): 75-79.
[10] 郭月琴, 孙志勇, 吴天骄, 等. 某钼铜硫多金属矿钼铜混合浮选分离研究[J]. 中国钼业, 2014(4): 32-37. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMY201404012.htm
GUO Y Q, SUN Z Y, WU T J, et al. Study on bulk flotation and separation of molybdenum and copper in a copper-molybdenum-sulfide polymetallic ore[J]. China Molybdenum Industry, 2014(4): 32-37. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMY201404012.htm
[11] 黄汝杰, 谢建宏, 张崇辉, 等. 陕西某含铅钼矿石选矿试验[J]. 金属矿山, 2013(7): 71-74. doi: 10.3969/j.issn.1001-1250.2013.07.019
HUANG R J, XIE J H, ZHANG C H, et al. Beneficiation tests of a molybdenum ore with lead from shaanxi[J]. Metal Mine, 2013(7): 71-74. doi: 10.3969/j.issn.1001-1250.2013.07.019
[12] 唐平宇, 田江涛, 葛敏, 等. 从钼铅硫化矿石中制备高品质钼精矿研究[J]. 矿冶工程, 2013, 33(1): 45-48. doi: 10.3969/j.issn.0253-6099.2013.01.012
TANG P Y, TIAN J T, GE M, et al. Preparing high quality molybdenum concentrate from Molybdenum-lead sulfide ores[J]. Mining And Metallurgical Engineering, 2013, 33(1): 45-48. doi: 10.3969/j.issn.0253-6099.2013.01.012
[13] 宋振国. 某难选钼矿选矿工艺技术研究[J]. 有色金属(选矿部分), 2012(1): 18-21. doi: 10.3969/j.issn.1671-9492.2012.01.005
SONG Z G. Technological study on mineral processing of a refractory molybdenum ore[J]. Nonferrous Metals(Mineral Processing Section), 2012(1): 18-21. doi: 10.3969/j.issn.1671-9492.2012.01.005
[14] 吴贤, 曹亮, 马光, 等. 含铅钼矿综合回收新工艺研究[J]. 中国钼业, 2012, 36(5): 7-11. doi: 10.3969/j.issn.1006-2602.2012.05.002
WU X, CAO L, MA G, et al. Research on new technologies for comprehensive utilization of lead-containing molybdenum[J]. China Molybdenum Industry, 2012, 36(5): 7-11. doi: 10.3969/j.issn.1006-2602.2012.05.002
[15] 卫亚儒, 王宇斌, 李继璧, 等. 某难选钼矿的选矿试验研究[J]. 中国钼业, 2011, 35(3): 18-21. doi: 10.3969/j.issn.1006-2602.2011.03.004
WEI Y R, WANG Y B, LI J B, et al. The processing flowsheet study on a complex molybdenum ore[J]. China Molybdenum Industry, 2011, 35(3): 18-21. doi: 10.3969/j.issn.1006-2602.2011.03.004
[16] 李苏玲, 白晓卿. 东沟钼矿应用磷诺克斯降铅实践[J]. 金属矿山, 2010(3): 183-184. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201003055.htm
LI S L, BAI X Q. Application of phosphoruspnox to lead reduction in Donggou molybdenum mine[J]. Metal Mine, 2010(3): 183-184. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS201003055.htm
[17] 周艳飞, 崔立凤. 江西某钼矿选矿试验研究[J]. 有色金属(选矿部分), 2012(3): 39-43. doi: 10.3969/j.issn.1671-9492.2012.03.011
ZHOU Y F, CUI L F. Research on mineral processing technology of a molybdenum ore in Jiangxi[J]. Nonferrous Metals(Mineral Processing Section), 2012(3): 39-43. doi: 10.3969/j.issn.1671-9492.2012.03.011
[18] 陈建华, 冯其明, 卢毅屏. 新型铜铅分离有机抑制剂ASC的研究[J]. 矿产保护与利用, 2000(5): 39-42. doi: 10.3969/j.issn.1001-0076.2000.05.010 http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=dcd67a7a-2611-46df-8c48-7ac9753cee3e
CHEN J H, FENG Q M, LU Y P. Research on a new organic depressant ASC for separation chalcopyrite and galena[J]. conservation and utilization of mineral resources, 2000(5): 39-42. doi: 10.3969/j.issn.1001-0076.2000.05.010 http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=dcd67a7a-2611-46df-8c48-7ac9753cee3e
[19] 蒋玉仁, 周立辉, 薛玉兰. 新型有机小分子抑制剂DPS的性能研究[J]. 有色金属工程, 2000(4): 33-40. doi: 10.3969/j.issn.2095-1744.2000.04.008
JIANG Y R, ZHOU L H, XUE Y L. Property and activity of a new low-molecular organic depressant-dps[J]. Nonferrous Metals, 2000(4): 33-40. doi: 10.3969/j.issn.2095-1744.2000.04.008
[20] 张泽强. 新型抑制剂在铜铅浮选分离中的应用[J]. 武汉化工学院学报, 1998, 20(4): 37-40. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG804.011.htm
ZHANG Z Q. The use of new depressant in copper lead separation by flotation[J]. Journal Of Wuhan Institute of Chemical Technology, 1998, 20(4): 37-40. https://www.cnki.com.cn/Article/CJFDTOTAL-WHHG804.011.htm
[21] 米丽平, 孙春宝, 李青, 等. 用组合抑制剂实现铜铅高效分离的试验研究[J]. 金属矿山, 2009(8): 53-56. doi: 10.3321/j.issn:1001-1250.2009.08.015
MI L P, SUN C B, LI Q, et al. Experimental Study on Copperlead Separation with the Combinatorial Depressant[J]. Metal Mine, 2009(8): 53-56. doi: 10.3321/j.issn:1001-1250.2009.08.015
[22] 魏明安, 孙传尧. 硫化铜, 铅矿物浮选分离研究现状及发展趋势[J]. 矿冶, 2008, 17(2): 6-16+33. https://www.cnki.com.cn/Article/CJFDTOTAL-KYZZ200802003.htm
WEI M A, SUN C Y. Review and development tendency of the copper and lead sulfides flotation separations[J]. Mining & Metallurgy, 2008, 17(2): 6-16+33. https://www.cnki.com.cn/Article/CJFDTOTAL-KYZZ200802003.htm
[23] 陈建华, 朱阳戈. 浮选体系矿物表面金属离子的半约束性质研究[J]. 中国矿业大学学报, 2021, 50(6): 1181-1188. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD202106015.htm
CHEN J H, ZHU Y G. Study of semi-constrained properties of metal ions on mineral surface of flotation system[J]. Journal of China University of Mining & Technology, 2021, 50(6): 1181-1188. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKD202106015.htm
[24] TAGGART A, GUIDICE G D, ZIEHL O. The case of the chemical theory of flotation[J]. Trans, AIME, 1934, 112: 348-381.
[25] COOK M A, NIXON J C. The theory of water-repellent films on solids formed by adsorption from aqueous solutions of heteropolar compounds. [J]. The Journal of Physical and Colloid Chemistry, 1950, 54(4): 445-459. doi: 10.1021/j150478a002
[26] SALEEB F, HANNA H. Correlation of adsorption, zeta potential, contact angle and flotation behaviour of calcium carbonate. J. Chem. ARE (United Arab Republic), 1969, 12: 229-236.
[27] SALAMY S, NIXON J. Reaction between a mercury surface and some flotation reagents: and electochemical study. I. Polarization curves[J]. Australian Journal of Chemistry, 1954, 7(2): 146-156. doi: 10.1071/CH9540146
[28] YE C, CHEN J, JIN G. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite[J]. Minerals Engineering, 2010, 23(14): 1120-1130. doi: 10.1016/j.mineng.2010.07.005
[29] CHEN J, YE C. A first-principle study of the effect of vacancy defects and impurities on the adsorption of O2 on sphalerite surfaces - sciencedirect[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 363(1/2/3): 56-63.
[30] CHEN J H, CHEN Y, LI Y Q. Quantum-mechanical study of effect of lattice defects on surface properties and copper activation of sphalerite surface[J]. 中国有色金属学报(英文版), 2010, 20(6): 10.
[31] LONG X, CHENY, CHEN J, et al. The effect of water molecules on the thiol collector interaction on the galena (PbS) and sphalerite (ZnS) surfaces: A DFT study[J]. Applied Surface Science, 2016, 389: 103-111.
[32] LONG X H. Adsorption of ethyl xanthate on ZnS(110) surface in the presence of water molecules: A DFT study[J]. Applied Surface Science A Journal Devoted to the Properties of Interfaces in Relation to the Synthesis & Behaviour of Materials, 2016, 370: 11-18.
[33] YIN Z, CHEN S, XU Z, et al. Flotation separation of molybdenite from chalcopyrite using an environmentally-efficient depressant L-cysteine and its adsoption mechanism[J]. Minerals Engineering, 2020, 156: 106438. doi: 10.1016/j.mineng.2020.106438
[34] YANG B, ZENG M, YAN H, et al. Tiopronin as a novel copper depressant for the selective flotation separation of chalcopyrite and molybdenite[J]. Separation and Purification Technology, 2021, 266(6): 118576.
[35] CHEN J, YANG X, LI Y, et al. Influences of electronic spin structures on the magnetic properties of Fe, Co and Ni ions and the adsorption of collectors[J]. Minerals Engineering, 2020, 154(6): 106405.
-



 下载:
下载: