Study on the Collection Properties of Lanthanide Metal-Benzohydroxamic Acid Organic Complexes for Fluorite and Calcite
-
摘要:
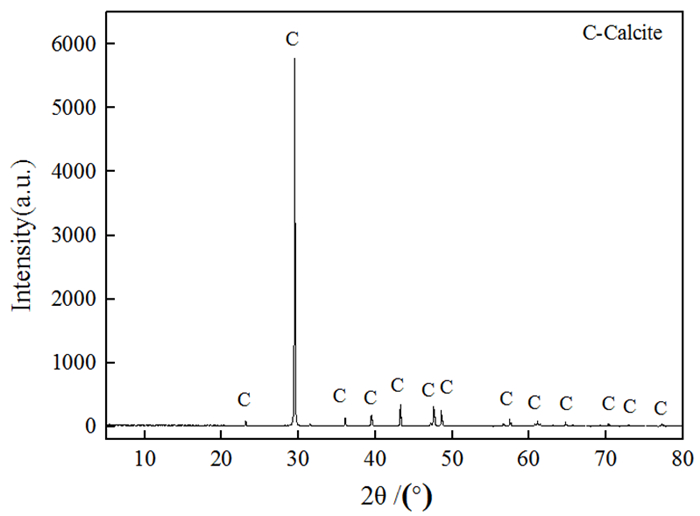
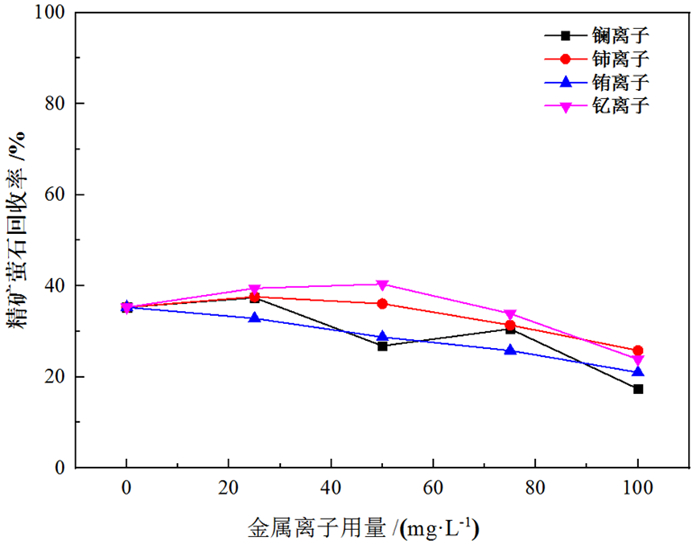
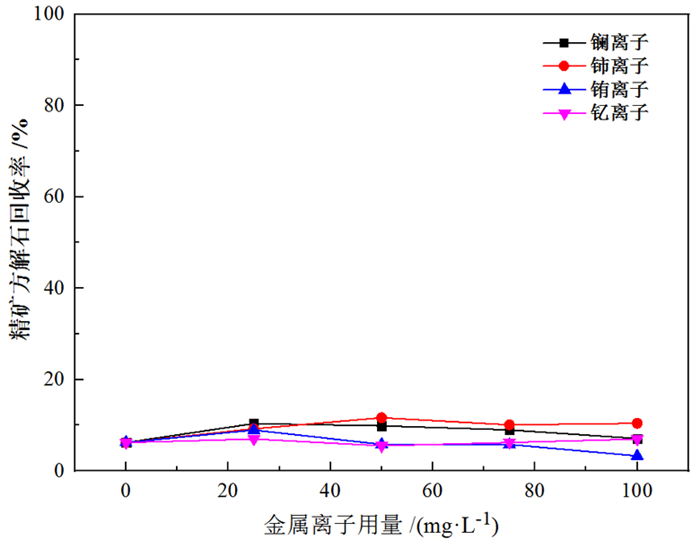
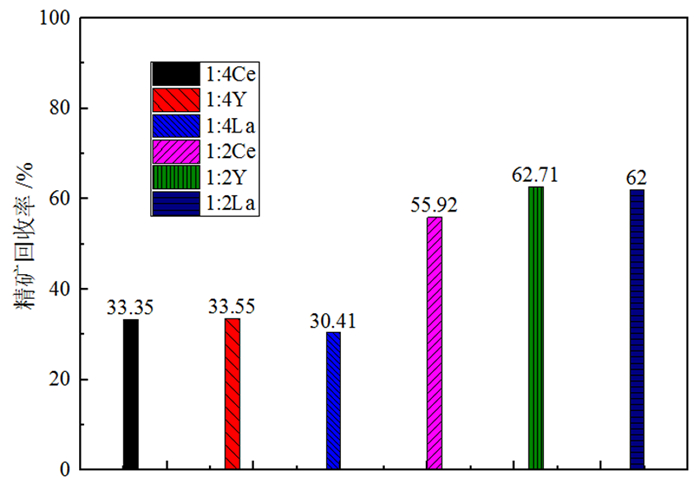
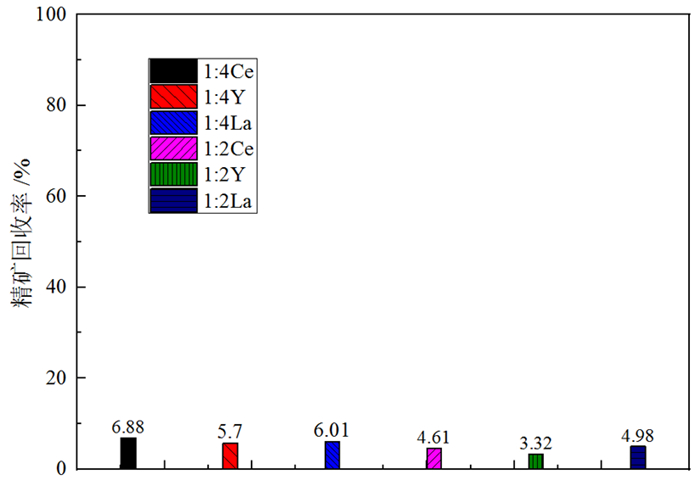
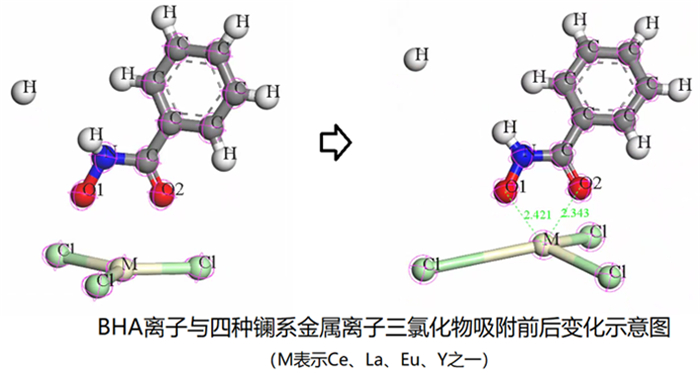
萤石、方解石等含钙矿物表面吸附位点同为钙质点, 导致脂肪酸、羟肟酸等常用氧化矿捕收剂对它们的选择性较差, 针对该问题提出利用含钙矿物阴离子的差异性来设计金属离子配合物捕收剂的思路。利用Ce-BHA配合物对萤石与方解石质量比1 GA6FA 1的人工混合矿进行一次粗选浮选试验, 最终可得萤石精矿CaF2品位78.92%、回收率74.77%, CaCO3品位11.08%、回收率24.23%, 分离效果良好。与单独使用BHA作捕收剂相比, 浮选精矿CaF2品位提高约26百分点, 回收率提高约50百分点, 表明该配合物捕收剂对萤石的选择性好。通过量子化学计算了多种镧系金属离子与苯甲羟肟酸(BHA)的作用情况及其在萤石、方解石表面的吸附行为, 结果显示最佳的配合物捕收剂组合为Ce-BHA, 与纯矿物浮选试验结果相符。
Abstract:The adsorption sites on the surface of calcium-bearing minerals are both calcium particles, which leads to poor selectivity of common oxidized ore collectors such as fatty acids and hydroxamic acids. In order to solve this problem, an idea of using the difference of anions of calcium-containing minerals to design metal ion complex collectors is proposed. Through a rough flotation test on the artificial mixed ore of fluorite and calcite with Ce-BHA complex, the final fluorite concentrate product was obtained with a grade of 78.92% and a recovery 74.77% of CaF2 while a grade of 11.08% and a recovery 24.23% of CaCO3. Compared with using BHA as collector alone, the grade of CaF2 of flotation concentrate was increased by about 26%, and the recovery was increased by nearly 50%, indicating that the complex collector has good selectivity for fluorite. The interaction of lanthanide metal ions with benzohydroxamic acid (BHA) and their adsorption behavior on the surface of fluorite and calcite were calculated by quantum chemistry. The results showed that the best collector combination of lanthanide metal ions was Ce-BHA, which was consistent with the results of pure mineral flotation test.
-
Key words:
- lanthanide metal complex /
- collector /
- quantum chemical calculation /
- fluorite /
- calcite /
- adsorption /
- flotation /
- benzohydroxamic acid
-

-
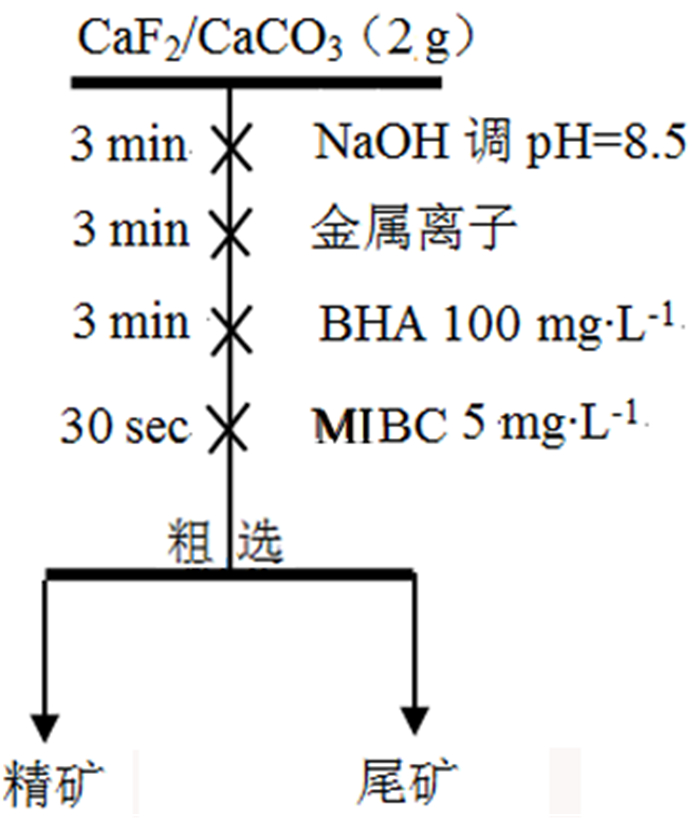
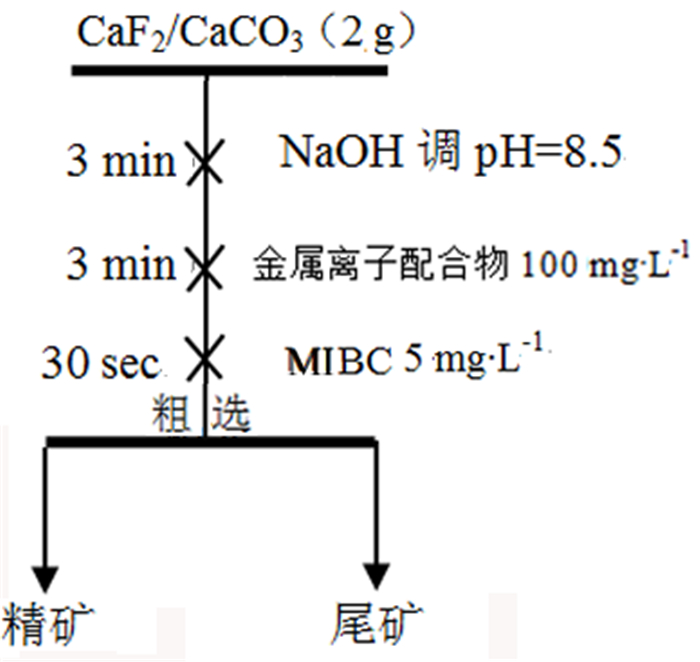
表 1 金属离子配合物/BHA浮选人工混合矿试验结果
Table 1. Results of flotation of mixed ores with metal ion complex/BHA
/% 试验条件 精矿产率 精矿萤石品位 精矿萤石回收率 精矿方解石品位 精矿方解石回收率 单独使用BHA 23.17 52.18 24.18 47.82 22.16 1:2Ce+BHA 47.37 78.92 74.77 21.08 19.97 1:2La+BHA 28.26 82.63 46.70 17.37 9.82 表 2 吸附前后原子键长及布居数变化情况
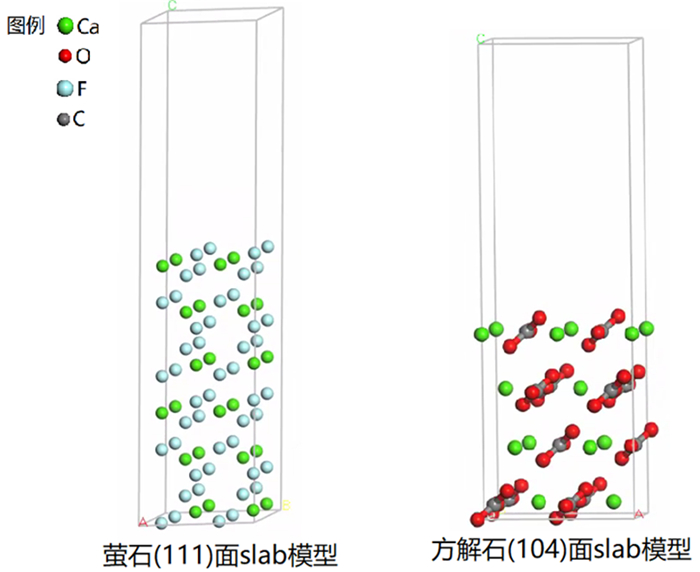
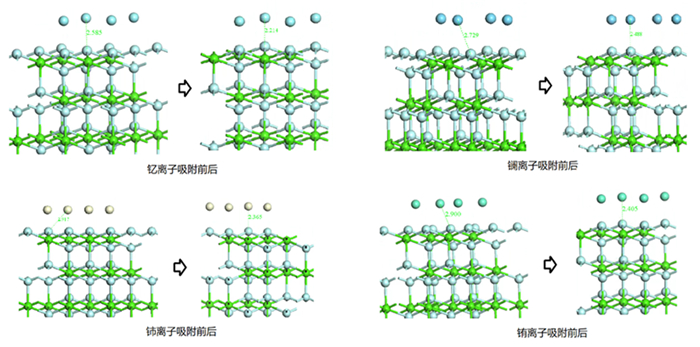
Table 2. Changes of atomic bond length and population before and after adsorption
成键关系 吸附前 吸附后 原子距离/Å 布居数 键长/Å 布居数 F-La 2.729 / 2.488 0.02 F-Ce 2.917 / 2.365 0.05 F-Eu 2.900 / 2.405 -0.01 F-Y 2.585 / 2.214 0.05 O-La 2.645 / 2.330 0.19 O-Ce 2.645 / 2.267 0.21 O-Eu 2.645 / 2.478 0.17 O-Y 2.645 / 2.143 0.24 表 3 吸附前后原子键长、布居数及吸附能变化情况
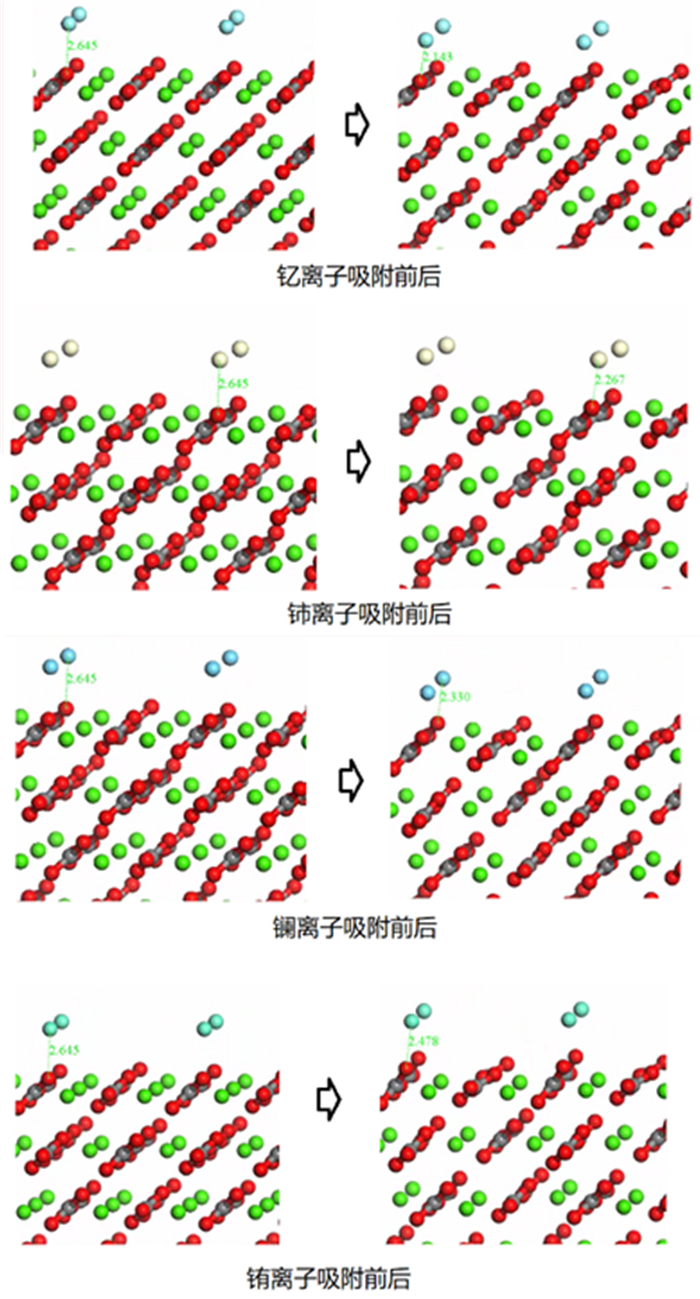
Table 3. Changes of atomic bond length, population and adsorption energy before and after adsorption
成键关系 吸附前 吸附后 单分子吸附能/eV 原子距离/Å 布居数 键长/Å 布居数 O1-La 2.645 / 2.217 0.33 -4.9581 O2-La 2.768 / 2.287 0.26 O1-Ce 2.645 / 2.421 0.28 -13.2072 O2-Ce 2.768 / 2.343 0.25 O1-Eu 2.645 / 2.209 0.26 -1.4514 O2-Eu 2.768 / 2.245 0.20 O1-Y 2.645 / 2.068 0.35 -4.2267 O2-Y 2.768 / 2.104 0.28 -
[1] 赵鹏, 郑厚义, 张新, 等. 中国萤石产业资源现状及发展建议[J]. 化工矿产地质, 2020, 42(2): 178-183. doi: 10.3969/j.issn.1006-5296.2020.02.014
ZHAO P, ZHENG H Y, ZHANG X, et al, Present situation and development suggestions of fluorite industry resources in China[J]. Geology of Chemical Minerals, 2020, 42(2): 178-183. doi: 10.3969/j.issn.1006-5296.2020.02.014
[2] 戴开明, 车长波, 王福良. 萤石资源勘查开发利用管理的建议[J]. 中国矿业, 2021, 289(9): 32-35. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKA202109007.htm
DAI K M, CHE C B, WANG F L. Suggestions on exploration, development and utilization management of fluorite resources[J]. China Mining Magazine, 2021, 289(9): 32-35. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGKA202109007.htm
[3] 于宝安, 杨勇, 王晓磊. 中国萤石矿床分类及其成矿规律[J]. 冶金管理, 2021(21): 90-91. https://www.cnki.com.cn/Article/CJFDTOTAL-YJGL202121048.htm
YU B A, YANG Y, WANG X L. Classification and metallogenic regularity of fluorite deposits in China[J]. China Steel Focus, 2021(21): 90-91. https://www.cnki.com.cn/Article/CJFDTOTAL-YJGL202121048.htm
[4] 林子华. 福建省萤石矿成矿地质特征[J]. 化工矿产地质, 2018, 40(3): 162-165. doi: 10.3969/j.issn.1006-5296.2018.03.005
LIN Z H. Metallogenic geological characteristics of fluorite deposits in Fujian province[J]. Geology of Chemical Minerals, 2018, 40(3): 162-165. doi: 10.3969/j.issn.1006-5296.2018.03.005
[5] 王敬元, 杜云, 田磊, 等. 湖南省萤石资源开发利用现状及找矿方向[J]. 国土资源导刊, 2020, 122(2): 26-30. doi: 10.3969/j.issn.1672-5603.2020.02.008
WANG J Y, Du Y, TIAN L, et al. The present situation of fluorite resources development and utilization and its prospecting direction in Hunan[J]. Land & Resources Herald, 2020, 122(2): 26-30. doi: 10.3969/j.issn.1672-5603.2020.02.008
[6] 杨开陆. 萤石/白云石浮选分离及机理研究[D]. 武汉: 武汉科技大学, 2018.
YANG K L. Study on flotation separation and mechanism of fluorite/dolomite[D]. Wuhan: Wuhan University of Science and Technology, 2018.
[7] 陶黎明, 王建军, 张晚佳, 等. 月桂酰肌氨酸钠选择性分离萤石和方解石及其作用机理[J]. 中国有色金属学报, 2021: 1-24.
TAO L M, WANG J J, ZHANG W J, et al. Selective separation of fluorite and calcite by sodium lauryl sarcosinate and its mechanism[J]. The Chinese Journal of Nonferrous Metals, 2021: 1-24.
[8] 胡岳华, 韩海生, 田孟杰, 等. 苯甲羟肟酸铅金属有机配合物在氧化矿浮选中的作用机理及其应用[J]. 矿产保护与利用, 2018(1): 42-47+53. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=850ab651-3250-4e85-8821-25be50b3d064
HU Y H, HAN H S, TIAN M J, et al. The application of metal-coordinated complexes in the flotation of oxide minerals and fundamental research of the adsorption mechanism[J]. Conservation and Utilization of Mineral Resources, 2018(1): 42-47+53. http://kcbh.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=850ab651-3250-4e85-8821-25be50b3d064
[9] 杨占峰, 李强, 王振江, 等. 白云鄂博矿床萤石型铁矿石中稀土分布规律研究[J]. 中国稀土学报, 2017, 168(4): 520-527. https://www.cnki.com.cn/Article/CJFDTOTAL-XTXB201704012.htm
YANG Z F, LI Q, WANG Z J, et al. Study on the distribution of rare earths in fluorite iron ore in Bayan Obo deposit[J]. Journal of the Chinese Society of Rare Earths, 2017, 168(4): 520-527. https://www.cnki.com.cn/Article/CJFDTOTAL-XTXB201704012.htm
[10] 王介良, 曹钊, 王建英, 等. 稀土矿浮选中Ce3+对萤石的活化作用机理[J]. 中国有色金属学报, 2018, 28(6): 1196-1203. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201806015.htm
WANG J L, CAO Z, WANG J Y, et al. Activation mechanism Ce3+ ions on fluorite of rare earth ore flotation[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(6): 1196-1203. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ201806015.htm
[11] 王鹏, 曹永丹, 王介良, 等. 辛基羟肟酸与硅酸钠对氟碳铈矿和萤石的浮选效果及量化计算[J]. 中国有色金属学报2021: 1-10.
WANG P, CAO Y D, WANG J L, et al. Flotation effect and quantum chemical calculation of octyl hydroxamic acid and sodium silicate on bastnaesite and fluorite[J]. The Chinese Journal of Nonferrous Metals, 2021: 1-10.
[12] 王鹏, 曹钊, 王介良, 等. 辛基羟肟酸与油酸的密度泛函理论计算及其对氟碳铈矿和萤石浮选效果的对比[J]. 中国有色金属学报, 2020, 30(8): 1974-1981. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ202008025.htm
WANG P, CAO Z, WANG J L, et al. Density functional theory calculation of octyl hydroxamic acid and oleic acid and comparison of flotation effects of fluorite and fluorite[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(8): 1974-1981. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXZ202008025.htm
[13] 徐诗佟, 黄海威, 任嗣利, 等. 萤石表面钙质点的加温溶出对其浮选行为的影响[J]. 金属矿山, 2022(3): 143-148. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202203018.htm
XU S T, HUANG H W, REN S L, et al. Influence of calcium point dissolution action on fluorite flotation behavior[J]. Metal Mine, 2022(3): 143-148. https://www.cnki.com.cn/Article/CJFDTOTAL-JSKS202203018.htm
[14] 史新章. 独居石和萤石晶体结构及表面吸附行为的第一性原理计算[D]. 包头: 内蒙古科技大学, 2020.
SHI X Z. First-principles calculation on the crystal structure and surface adsorption behavior of monazite and fluorite[D]. Baotou: Inner Mongolia University of Science and Technology, 2020.
[15] 柴汝宽, 刘月田, 杨莉, 等. 两种不同极性有机小分子在方解石(104)面吸附的密度泛函研究[J]. 计算物理, 2020, 37(2): 221-230. https://www.cnki.com.cn/Article/CJFDTOTAL-JSWL202002011.htm
CHAI L K, LIU Y T, YANG L, et al. Density functional study on the adsorption of two small organic molecules with different polarity on calcite (104) surface[J]. Chinese Journal of Computational Physics, 2020, 37(2): 221-230. https://www.cnki.com.cn/Article/CJFDTOTAL-JSWL202002011.htm
-



 下载:
下载: