Chemical Modification of Natural Magnetite and Its Application in Arsenic Removal from a Water Environment
-
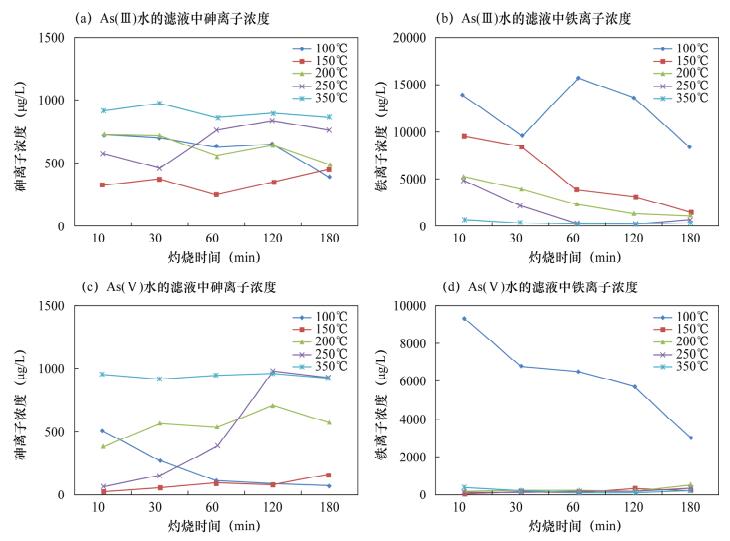
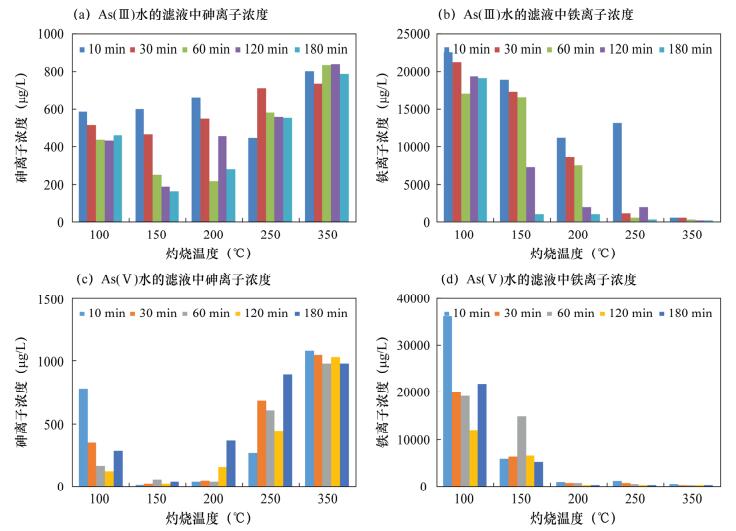
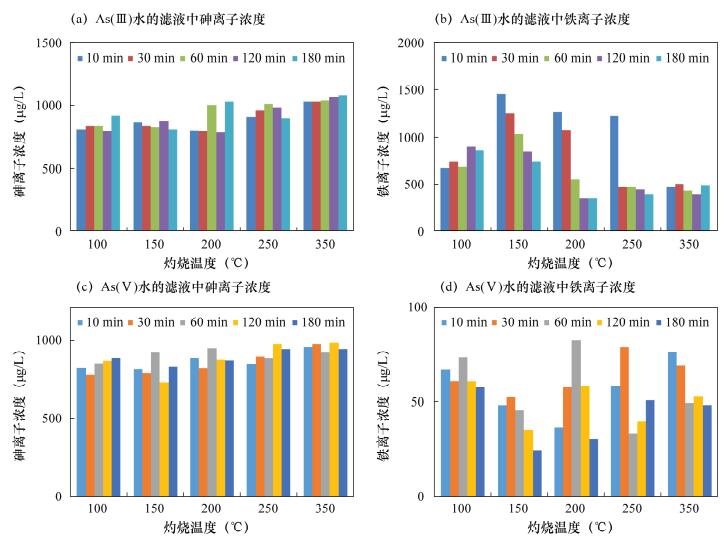
摘要: 铁氧化物及其复合氧化物(如菱铁矿、水铁矿)的表面电荷高、比表面积大,在特定条件下对亚砷酸盐和砷酸盐有较强的结合能力和亲和性,以铁氧化物作为吸附剂处理高砷水已经成为研究热点之一。天然磁铁矿的主要成分为Fe3O4,但其本身活性较弱,直接应用于处理高砷水的除砷率低。本文对天然磁铁矿采取酸化、碱化、不同温度灼烧、不同灼烧时间等简易的方法进行改性,达到有效去除水中砷的目的。实验结果表明:经0.5 mol/L盐酸浸泡、150℃灼烧10 min的改性磁铁矿分别处理As(Ⅲ)溶液和As(Ⅴ)溶液时,As(Ⅴ)去除率达98%,吸附能力显著增强,达到预期目标;溶液中As(Ⅲ)浓度从1000 μg/L下降到250 μg/L,去除率达75%,即As吸附能力明显优于未改性的天然磁铁矿,与其他改性铁矿除砷能力相近,而改性方法更加简便、易行。本文研究的改性天然磁铁矿吸附剂为控制高砷水的砷含量提供了一种切实可用的吸附材料。Abstract: Iron oxides and their complex oxides such as siderite and ferrihydrite have high surface charge and specific surface area, which have strong binding capacity and affinity for arsenite and arsenate under specific conditions. Natural magnetite, mainly consisting of Fe3O4, has low activity itself, and is not efficient for direct treatment of high arsenic water. Reported in this paper, natural magnetite is modified by acidification, alkalization, and calcined at different temperatures for different periods, in order to enhance arsenic removal from water. Experimental results show that the modified magnetite acidified in 0.5 mol/L hydrochloric acid and calcined at 150℃ for 10 min, has high removal efficiencies for both As (Ⅲ) and As (Ⅴ). Removal efficiency was 98% for As (Ⅴ), whereas the removal efficiency was 75% for As (Ⅲ) with concentration decreases from 1000 μg/L to 250 μg/L (in Fig.2). The modified natural magnetite has stronger adsorption capacity for As than natural magnetite, and is identical to other modified iron minerals. In addition, the modification method is simple and convenient. Therefore, the modified natural magnetite would be a potential adsorbent for controlling arsenic content in high arsenic water.
-
Key words:
- natural magnetite /
- chemical modification /
- water environment /
- arsenic adsorption
-

-
表 1 含砷水在不同碱性处理磁铁矿作用下的砷含量和铁含量
Table 1. Contents of arsenic and iron in alkali-treated magnetite ore before and after the removal of As
改性条件 含砷水滤液中离子浓度含量 (μg/L) As (Ⅲ) 铁离子 As (Ⅴ) 铁离子 空白 1051 2.6 1031 3.2 未处理磁铁矿 1039 3.8 1048 2.9 0.1 mol/L碱处理后,
150℃灼烧10 min磁铁矿1056 3.1 1051 3.3 0.5 mol/L碱处理后,
150℃灼烧10 min磁铁矿1026 3.2 1038 3.4 1.0 mol/L碱处理后,
150℃灼烧10 min磁铁矿1044 2.3 1024 2.6 表 2 不同条件处理磁铁矿对含砷水的砷去除率
Table 2. Removal rate of As-containing water treated with modified magnetite
改性条件 水体中砷的去除率 (%) As (Ⅲ) As (Ⅴ) 不同浓度碱处理后,
150℃灼烧10 min磁铁矿0 0 0.1 mol/L盐酸处理后,
150℃灼烧10 min磁铁矿13.2 18.6 0.5 mol/L盐酸处理后,
150℃灼烧10 min磁铁矿66.9 98.1 1.0 mol/L盐酸处理后,
150℃灼烧10 min磁铁矿39.7 98.4 -
[1] 陈保卫, Chris L X.中国关于砷的研究进展[J].环境化学, 2011, 30(11):1936-1943. http://www.cnki.com.cn/Article/CJFDTOTAL-HJHX201111018.htm
Chen B W, Chris L X.Recent progress in arsenic research in China[J].Environmental Chemistry, 2011, 30(11):1936-1943. http://www.cnki.com.cn/Article/CJFDTOTAL-HJHX201111018.htm
[2] 张玉玺, 向小平, 张英, 等.云南阳宗海砷的分布与来源[J].环境科学, 2012, 33(11):3768-3777. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201211013.htm
Zhang Y X, Xiang X P, Zhang Y, et al.Distribution and sources of arsenic in Yangzonghai Lake, China[J]. Environmental Science, 2012, 33(11):3768-3777. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201211013.htm
[3] 张楠, 韦朝阳, 杨林生.淡水湖泊生态系统中砷的赋存与转化行为研究进展[J].生态学报, 2013, 33(2):337-347. doi: 10.5846/stxb http://www.cnki.com.cn/Article/CJFDTOTAL-STXB201302004.htm
Zhang N, Wei C Y, Yang L S.Advance in research on the occurrence and transformation of arsenic in the freshwater lake ecosystem[J].Acta Ecologica Sinica, 2013, 33(2):337-347. doi: 10.5846/stxb http://www.cnki.com.cn/Article/CJFDTOTAL-STXB201302004.htm
[4] 郑红, 梁树平, 曹燕飞, 等.赤泥与13X沸石混合使用去除废水中砷[J].岩矿测试, 2006, 25(3):239-242. http://www.ykcs.ac.cn/ykcs/ch/reader/view_abstract.aspx?file_no=20060379&flag=1
Zheng H, Liang S P, Cao Y F, et al.Dearsenication effects of 13X zeolite mixed with red mud[J].Rock and Mineral Analysis, 2006, 25(3):239-242. http://www.ykcs.ac.cn/ykcs/ch/reader/view_abstract.aspx?file_no=20060379&flag=1
[5] Sun H J, Rathinasabapathi B, Wu B, et al.Arsenic and selenium toxicity and their interactive effects in humans[J].Environment International, 2014, 69(4):148-158.
[6] 贾永锋, 郭华明.高砷地下水研究的热点及发展趋势[J].地球科学进展, 2013, 28(1):51-61. http://www.cnki.com.cn/Article/CJFDTOTAL-DXJZ201301007.htm
Jia Y F, Guo H M.Hot topics and trends in the study of high arsenic groundwater[J].Advances in Earth Science, 2013, 28(1):51-61. http://www.cnki.com.cn/Article/CJFDTOTAL-DXJZ201301007.htm
[7] 杨芬, 朱晓东, 韦朝阳.陆地水环境中砷的迁移转化[J].生物学杂志, 2015, 34(5):1448-1455. http://www.cnki.com.cn/Article/CJFDTOTAL-STXZ201505039.htm
Yang F, Zhu X D, Wei C Y.A overview on the process and mechanism of arsenic transformation and transportation in aquatic environment[J].Chinese Journal of Ecology, 2015, 34(5):1448-1455. http://www.cnki.com.cn/Article/CJFDTOTAL-STXZ201505039.htm
[8] 吴万富, 徐艳, 史德强, 等.我国河流湖泊砷污染现状及除砷技术研究进展[J].环境科学与技术, 2015, 38(6):190-197.
Wu W F, Xu Y, Shi D Q, et al.The arsenic pollution status of the rivers and lakes in China and the research progress on arsenic removal techniques[J].Environmental Science & Technology, 2015, 38(6):190-197.
[9] Baig S A, Sheng T, Hu Y, et al.Arsenic removal from natural water using low cost granulated adsorbents:A review[J].CLEAN-Soil, Air, Water, 2015, 43(1):13-26. doi: 10.1002/clen.201200466
[10] Sears M E, Kerr K J, Bray R I.Arsenic, cadmium, lead, and mercury in sweat:A systematic review[J].Journal of Environmental & Public Health, 2012, doi:10.1155/2012/184745.
[11] Leiva E D, Rámila C D P, Vargas I T, et al.Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed[J].Science of the Total Environment, 2014, 466-467(1):490-502.
[12] 席北斗, 王晓伟, 霍守亮, 等.纳滤膜技术在地下水除砷应用中的研究进展[J].环境工程学报, 2012, 6(2):353-360. http://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201202001.htm
Xi B D, Wang X W, Huo S L, et al.Investigation progress of arsenic removal from groundwater by nanofiltration membrane technology[J].Chinese Journal of Environmental Engineering, 2012, 6(2):353-360. http://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ201202001.htm
[13] Bordoloi S, Nath S K, Gogoi S, et al.Arsenic and iron removal from groundwater by oxidation coagulation at optimized pH:Laboratory and field studies[J].Journal of Hazardous Materials, 2013, 260C (1):618-626. https://www.researchgate.net/publication/245537874_Arsenic_and_iron_removal_from_groundwater_by_oxidation-coagulation_at_optimized_pH_Laboratory_and_field_studies
[14] Klerk R J D, Jia Y, Daenzer R, et al.Continuous circuit coprecipitation of arsenic (Ⅴ) with ferric iron by lime neutralization:Process parameter effects on arsenic removal and precipitate quality[J].Hydrometallurgy, 2012, 111-112(1):65-72. https://www.researchgate.net/profile/Mario_Gomez4/publication/251562405_Continuous_Circuit_Co-Precipitation_of_ArsenicV_with_Ferric_Iron_by_Lime_Neutralization_Process_Parameter_Effects_on_Arsenic_Removal_and_Precipitate_Quality/links/56259e4a08aeedae57daef36.pdf
[15] Abejón A, Garea A, Irabien A.Arsenic removal from drinking water by reverse osmosis:Minimization of costs and energy consumption[J].Separation & Purification Technology, 2015, 144:46-53. https://www.researchgate.net/publication/272887349_Arsenic_removal_from_drinking_water_by_reverse_osmosis_Minimization_of_costs_and_energy_consumption
[16] Guo H, Wen D, Liu Z, et al.A review of high arsenic groundwater in Mainland and Taiwan, China:Distribution, characteristics and geochemical processes[J].Applied Geochemistry, 2014, 41(1):196-217.
[17] Guo H M, Stüben D, Berner Z.Adsorption of arsenic (Ⅲ) and arsenic (Ⅴ) from groundwater using natural siderite as the adsorbent[J].Journal of Colloid and Interface Science, 2007, 315(1):47-53. doi: 10.1016/j.jcis.2007.06.035
[18] Guo H, Ren Y, Liu Q, et al.Enhancement of arsenic adsorption during mineral transformation from siderite to goethite:Mechanism and application[J].Environmental Science & Technology, 2013, 47(2):1009-1016. https://www.researchgate.net/profile/Huaming_Guo/publication/233955963_Enhancement_of_Arsenic_Adsorption_during_Mineral_Transformation_from_Siderite_to_Goethite_Mechanism_and_Application/links/0c960538c7c63b91cb000000.pdf
[19] Zhang G, Liu H, Qu J, et al.Arsenate uptake and arsenite simultaneous sorption and oxidation by Fe-Mn binary oxides:Influence of Mn/Fe ratio, pH, Ca2+, and humic acid[J].Journal of Colloid & Interface Science, 2012, 366(1):141-146.
[20] 赵凯, 郭华明, 李媛, 等.天然菱铁矿改性及强化除砷研究[J].环境科学, 2012, 33(2):459-468. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201202021.htm
Zhao K, Guo H M, Li Y, et al.Modification of natural siderite and enhanced adsorption of arsenic[J].Environmental Science, 2012, 33(2):459-468. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201202021.htm
[21] Tuna A Ö A, Özdemir E, Simsek E B, et al.Optimization of process parameters for removal of arsenic using activated carbon-based iron-containing adsorbents by response surface methodology[J].Water, Air & Soil Pollution, 2013, 224(9):1685-1699. https://www.researchgate.net/publication/257804899_Optimization_of_Process_Parameters_for_Removal_of_Arsenic_Using_Activated_Carbon-Based_Iron-Containing_Adsorbents_by_Response_Surface_Methodology
[22] 刘春华, 郭华明, 郑伟, 等.天然磁铁矿吸附-电感耦合等离子体质谱测定砷[J].分析化学, 2011, 39(1):115-119. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX201101029.htm
Liu C H, Guo H M, Zheng W, et al.Natural magnetite solid phase extraction-inductively coupled plasma mass spectrometry for determination of arsenic in water[J].Chinese Journal of Analytical Chemistry, 2011, 39(1):115-119. http://www.cnki.com.cn/Article/CJFDTOTAL-FXHX201101029.htm
[23] Giménez J, Martínez M, De P J, et al.Arsenic sorption onto natural hematite, magnetite, and goethite[J].Journal of Hazardous Materials, 2007, 141(3):575-580. doi: 10.1016/j.jhazmat.2006.07.020
[24] Pokhrel D, Viraraghavan T.Arsenic removal from an aqueous solution by a modified fungal biomass[J].Water Research, 2006, 40(3):549-552. doi: 10.1016/j.watres.2005.11.040
-



 下载:
下载: