Simultaneous Determination of Chromium(Ⅲ) and Chromium(Ⅵ) in Water by the First Derivative Spectrophotometric Method
-
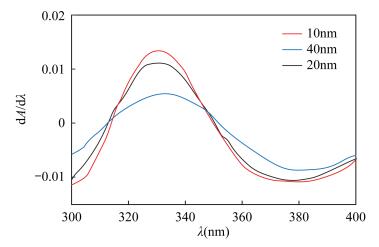
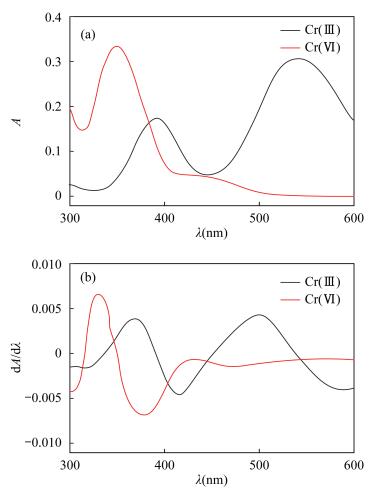
摘要: 环境水样中的铬通常以Cr(Ⅲ)和Cr(Ⅵ)的形态存在,不同价态的铬产生不同的生理作用,对不同价态铬进行准确分析是必要的。目前Cr(Ⅲ)和Cr(Ⅵ)的测定方法大多数是先分离后再测定,或先测定出Cr(Ⅲ)或Cr(Ⅵ),然后通过氧化或还原测定铬的总量,再差减法计算出另一价态铬的含量,此操作复杂,并且在处理过程中易导致价态的改变,误差较大,精确度难以保证。本文用一阶导数分光光度法消除了Cr(Ⅲ)对Cr(Ⅵ)干扰,能同时测定Cr(Ⅲ)或Cr(Ⅵ)的含量。混合水样中加入显色剂EDTA-2Na,调节溶液pH在3~3.5,恒温水浴70℃条件保持加热15min,测定吸光度,导数间隔因子为10nm求一阶导数,在波长330nm处Cr(Ⅵ)吸光度一阶导数有最大值,而在此波长处Cr(Ⅲ)-EDTA络合物吸光度一阶导数值为0,可用一阶导数分光光度法测定Cr(Ⅵ)的含量,在Cr(Ⅲ)-EDTA的最大吸收波长543nm处测溶液吸光度,直接测定Cr(Ⅲ)的含量。在优化实验条件下,Cr(Ⅲ)线性回归方程为A=0.0036ρ-0.0002(r2=0.9999),线性范围为0~120mg/L,检出限为0.006mg/L;Cr(Ⅵ)线性回归方程为D=0.00072ρ-0.00013(D为一阶导数值,r2=0.9996),线性范围为0~100mg/L,检出限为0.005mg/L。Cr(Ⅲ)和Cr(Ⅵ)加标回收率为97.8%~102.6%。该方法能够满足废水中Cr(Ⅲ)和Cr(Ⅵ)分析测试要求。Abstract:
OBJECTIVES Chromium is usually found as Cr(Ⅲ) and Cr(Ⅵ) in environmental water samples. The different valences of chromium produce different physiological function, thus it is necessary to analyze the different valences of chromium accurately. At present, the contents of Cr(Ⅲ) and Cr(Ⅵ) are mostly determined after separation or the content of Cr(Ⅲ) or Cr(Ⅵ) is measured first, then the total content of Cr is determined after oxidation or reduction. The content of another valence of chromium is then calculated by the subtraction method. The available method needs a complex procedure. Moreover, the valence of Cr is easily modified during the sample treatment, resulting in large error and low precision. OBJECTIVES To find a simple and accurate method for determination of Cr(Ⅲ) and Cr(Ⅵ). METHODS The first derivative spectrophotometric method was used for simultaneously determining of Cr(Ⅲ) and Cr(Ⅵ), which eliminated the interference of Cr(Ⅲ) on Cr(Ⅵ). RESULTS Chromogenic reagent EDTA-2Na was added to the mixed water sample in a 70℃water-bath at pH 3-3.5 for 15min, and the absorbance was measured. When the derivative interval factor was 10nm, the first derivative value of absorption for Cr(Ⅵ) was the maximum at 330nm wavelength and the value for Cr(Ⅲ)-EDTA was zero. The concentration of Cr(Ⅵ) can be obtained by the first derivative spectrophotometric method, whereas Cr(Ⅲ) can be determined directly at the maximum wavelength of 543nm. Under the optimal conditions, the concentration range was 0-100mg/L for Cr(Ⅵ) and 0-120mg/L for Cr(Ⅲ). The equation of linear regression for Cr(Ⅲ) was A=0.0036ρ-0.0002 (r2=0.9999), for Cr(Ⅵ) was D=0.00072ρ-0.00013 (r2=0.9996). and the limit of detection was 0.005mg/L for Cr(Ⅵ) and 0.006mg/L for Cr(Ⅲ). The recoveries for Cr(Ⅲ) and Cr(Ⅵ) were 97.8%-102.6%. Conclusion The method meets the requirements for analyzing waste water. -

-
表 1 反应时间及温度对Cr(Ⅲ)吸光度的影响
Table 1. Effect of reaction time and temperature on the absorbance of Cr(Ⅲ)
反应温度
(℃)吸光度 5min 10min 15min 20min 25min 30min 40 0.0104 0.0384 0.0489 0.0567 0.0611 0.0643 50 0.0452 0.0582 0.0682 0.0687 0.0701 0.0758 60 0.0730 0.0758 0.0772 0.0784 0.0786 0.0795 70 0.0756 0.0779 0.0780 0.0783 0.0791 0.0796 80 0.0751 0.0781 0.0791 0.0802 0.0805 0.0806 表 2 干扰离子对Cr(Ⅲ)和Cr(Ⅵ)测定的影响
Table 2. Effect of interferencing ions on the determination of Cr(Ⅲ) and Cr(Ⅵ)
干扰物质 干扰物质浓度(mg/L) 混合溶液浓度(mg/L) Cr(Ⅲ)测得量(mg/L) Cr(Ⅵ)测得量(mg/L) 相对误差(%) Cr(Ⅲ) Cr(Ⅵ) Cr(Ⅲ) Cr(Ⅵ) Mg2+ 800 4.0 4.0 5.57 4.00 39.3 0 500 4.0 4.0 4.16 4.14 4.0 3.5 Al3+ 200 4.0 4.0 4.96 4.14 24.0 3.5 100 4.0 4.0 4.08 3.98 2.0 0.5 Cu2+ 100 4.0 4.0 4.32 4.40 8.0 10 50 4.0 4.0 4.20 4.16 5.0 4.0 Co3+ 200 4.0 4.0 4.78 4.16 19.5 4.0 100 4.0 4.0 4.06 4.02 1.5 0.5 Pb2+ 200 4.0 4.0 4.55 4.14 13.8 3.5 100 4.0 4.0 4.06 4.12 1.5 3.0 Ni2+ 100 4.0 4.0 5.26 4.28 31.5 7.0 50 4.0 4.0 4.13 4.12 3.2 3.0 Fe3+ 40 4 4 4.63 4.80 15.8 20 20 4 4 4.15 4.16 3.8 4.0 表 3 样品中Cr(Ⅲ)和Cr(Ⅵ)的测定结果及加标回收率
Table 3. Analytical results and spiked recovery of Cr(Ⅲ) and Cr(Ⅵ) in samples
实际样品 铬形态 测定值
(mg/L)加标量
(mg/L)总回收量
(mg/L)回收率
(%)电镀废液 Cr(Ⅲ)
Cr(Ⅵ)5.05
8.895.0
5.09.91
13.9698.2
101.4废铬酸洗液 Cr(Ⅲ) 16.07 5.0 21.20 102.6 Cr(Ⅵ) 9.14 5.0 14.03 97.8 -
[1] 王蓉, 张丽萍, 邹时英.偶氮胂Ⅲ褪色光度法测定土壤中的铬[J].岩矿测试, 2011, 30(2):230-232. http://www.ykcs.ac.cn/article/id/ykcs_20110224
Wang R, Zhang L P, Zou S Y.Determination of chromium in soil samples by arsenazo Ⅲ fading spectrophotometry[J].Rock and Mineral Analysis, 2011, 30(2):230-232. http://www.ykcs.ac.cn/article/id/ykcs_20110224
[2] Chen S J, Zhang X S, Yu L Y, et al.Simultaneous determination of Cr(Ⅲ) and Cr(Ⅵ) in tannery wastewater using low pressure ion chromatography combined with flow injection spectrophotometry[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2012, 88:49-55. doi: 10.1016/j.saa.2011.11.053
[3] Ouejhani A, Hellal F, Dachraoui M, et al.Application of doehlert matrix to the study of electrochemical oxidation of Cr(Ⅲ) to Cr(Ⅵ) in order to recover chromium from wastewater tanning baths[J].Journal of Hazardous Materials, 2008, 157(2/3):423-431. http://www.sciencedirect.com/science/article/pii/S0304389408000447
[4] Tardif S, Cipullo S, Sø H U, et al.Factors governing the solid phase distribution of Cr, Cu and As in contaminated soil after 40 years of ageing[J].Science of the Total Environment, 2019, 652:744-754. doi: 10.1016/j.scitotenv.2018.10.244
[5] Diao Z H, Du J J, Jiang D, et al.Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge derived biochar immobilized nanoscale zero valent iron:Coexistence effect and mechanism[J].Science of the Total Environment, 2018, 642:505-515. doi: 10.1016/j.scitotenv.2018.06.093
[6] 张杰芳, 闫玉乐, 夏承莉, 等.微波碱消解-电感耦合等离子体发射光谱法测定煤灰中的六价铬[J].岩矿测试, 2017, 36(1):45-51. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2017.01.007
Zhang J F, Yan Y L, Xia C L, et al. Determination of Cr(Ⅵ) in coal ash by microwave alkaline digestion and inductively coupled plasma-optical emission spectrometry[J].Rock and Mineral Analysis, 2017, 36(1):45-51. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2017.01.007
[7] 李冰茹, 杜远芳, 王北洪, 等.食品中总铬和铬形态分析的前处理技术概述[J].食品安全质量检测学报, 2018, 9(9):2056-2062. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spaqzljcjs201809013
Li B R, Du Y F, Wang B H, et al.Research progress on the pretreatment techniques in the analysis of total chromium and speciation chromium in food[J].Journal of Food Safety and Quality, 2018, 9(9):2056-2062. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spaqzljcjs201809013
[8] Economou-Eliopoulos M, Megremi Ⅰ, Vasilatos C. Geochemical constraints on the sources of Cr(Ⅵ) contamination in waters of Messapia (Central Evia) Basin[J].Applied Geochemistry, 2017, 84:13-25. doi: 10.1016/j.apgeochem.2017.05.015
[9] 安茂国, 赵庆令, 谭现锋, 等.化学还原-稳定化联合修复铬污染场地土壤的效果研究[J].岩矿测试, 2019, 38(2):204-211. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201806040068
An M G, Zhao Q L, Tan X F, et al.Research on the effect of chemical reduction-stabilization combined remediation of Cr-contaminated soil[J].Rock and Mineral Analysis, 2019, 38(2):204-211. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201806040068
[10] Mulware S J.Trace elements and carcinogenicity:A subject in review[J].Biotechnology, 2013, 3(2):85-96. http://link.springer.com/article/10.1007/s13205-012-0072-6
[11] Clemention M, Shi X L, Zhang Z.Oxidative stress and metabolic reprogramming in Cr(Ⅵ) carcinogenesis[J].Current Opinion in Toxicology, 2018, 8:20-27. doi: 10.1016/j.cotox.2017.11.015
[12] 陈思涵, 彭璨, 陈云生, 等.分光光度法测定胶囊中Cr(Ⅲ)和Cr(Ⅵ)[J].中国卫生检验杂志, 2015, 25(1):18-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgwsjyzz201501005
Chen S H, Peng C, Chen Y S, et al.Detection of Cr(Ⅲ) and Cr(Ⅵ) in capsule with spectrophotometry[J].Chinese Journal of Health Laboratory Technology, 2015, 25(1):18-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgwsjyzz201501005
[13] 张冀飞, 高欣, 杨晓兵.高效液相色谱-电感耦合等离子体质谱联用测定儿童玩具中痕量铬(Ⅲ)与铬(Ⅵ)[J].分析测试学报, 2015, 34(2):232-236. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxcsxb201502019
Zhang J F, Gao X, Yang X B.Determination of chromium(Ⅲ) and chromium(Ⅵ) in children's toys using high performance liquid chromatography-inductively coupled plasma mass spectrometry[J].Journal of Instrumental Analysis, 2015, 34(2):232-236. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxcsxb201502019
[14] 林莉, 郑翊, 卫碧文, 等.IC-ICP-MS联用法测定玩具材料中可迁移的六价铬与三价铬[J].分析试验室, 2013, 32(8):82-85. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys201308018
Lin L, Zheng Y, Wei B W, et al.Simultaneous determination of migratory chromium species in toy materials by ion chromatography and ICP-MS[J].Chinese Journal of Analysis Laboratory, 2013, 32(8):82-85. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys201308018
[15] 闫美, 谢晨星, 朱智惠, 等.保健食品中三价铬与六价铬的分离与测定[J].食品研究与开发, 2016, 37(7):171-175. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spyjykf201607042
Yan M, Xie C X, Zhu Z H, et al.The research on the conditions of hexavalent chromium convert to trivalent chromium in healthy food[J].Food Research and Development, 2016, 37(7):171-175. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spyjykf201607042
[16] 刘明理, 曹进, 丁宏, 等.液相色谱-质谱法测定保健食品中的三价铬及六价铬含量[J].食品安全质量检测学报, 2017, 8(7):2465-2470. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spaqzljcjs201707016
Liu M L, Cao J, Ding H, et al.Determination of trivalent and hexavalent chromium in healthy food by liquid chromatography-mass spectrometry[J].Journal of Food Safety and Quality, 2017, 8(7):2465-2470. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spaqzljcjs201707016
[17] 庞艳华, 刘名扬, 刘淑艳, 等.反相离子对色谱-电感耦合等离子体质谱法测定化妆品中不同形态的铬[J].色谱, 2011, 29(10):1027-1030. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sp201110014
Pang Y H, Liu M Y, Liu S Y, et al.Simultaneous determination of chromium speciation in cosmetics using reversed-phase ion-pair chromatography-inductively coupled plasma mass spectrometry[J].Chinese Journal of Chromatography, 2011, 29(10):1027-1030. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sp201110014
[18] 吴思霖, 王欣美, 潘晨, 等.高效液相色谱-电感耦合等离子体质谱联用测定化妆品中六价铬与三价铬[J].分析测试学报, 2019, 38(6):724-727. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxcsxb201906015
Wu S L, Wang X M, Pan C, et al.Determination of chromium(Ⅵ) and chromium(Ⅲ) in cosmetics by high performance liquid chromatography-inductively coupled plasma mass spectrometry[J].Journal of Instrumental Analysis, 2019, 38(6):724-727. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxcsxb201906015
[19] 张保科, 王蕾, 马生凤.电感耦合等离子体质谱法测定含气天然矿泉水中的铬[J].岩矿测试, 2013, 32(4):568-571. http://www.ykcs.ac.cn/article/id/f4ece52c-7679-4883-a2ff-e57adb9b0536
Zhang B K, Wang L, Ma S F.Quantification of Cr in natural sparkling mineral waters by inductively coupled plasma-mass spectrometry[J].Rock and Mineral Analysis, 2013, 32(4):568-571. http://www.ykcs.ac.cn/article/id/f4ece52c-7679-4883-a2ff-e57adb9b0536
[20] 邢夏, 徐进力, 何晓辉, 等.石墨炉原子吸收光谱法测定地球化学样品中微量铬[J].岩矿测试, 2011, 30(3):333-336. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ykcs201103019
Xing X, Xu J L, He X H, et al.Determination of trace chromium in geochemical samples by graphite furnace atomic absorption spectrometry[J].Rock and Mineral Analysis, 2011, 30(3):333-336. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ykcs201103019
[21] Sadeghi S, Moghaddam A Z.Chromium speciation using task specific ionic liquid/aqueous phase biphasic system combined with flame atomic absorption spectrometry[J].Journal of Molecular Liquids, 2016, 221:798-804. doi: 10.1016/j.molliq.2016.06.056
[22] 卢菊生, 徐佳佳, 田久英, 等.微乳相萃取分离富集-原子吸收光谱法分析铬形态[J].应用化学, 2010, 27(10):1230-1234. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yyhx201010022
Lu J S, Xu J J, Tian J Y, et al.Speciation determination of chromium by atomic absorption spectrometry with separation of microemulsion extraction[J].Chinese Journal of Applied Chemistry, 2010, 27(10):1230-1234. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yyhx201010022
[23] Tafti E N, Dadfarnia S, Shabani A M H.Supramolecular dispersive liquid-liquid microextraction-based solidification of floating organic drops combined with electrothermal atomic absorption spectrometry for determination of chromium species[J].International Journal of Environmental Analytical Chemistry, 2017, 97(5):444-445. doi: 10.1080/03067319.2017.1321743
[24] Monasterio R P, Lascalea G E, Martínez L D, et al. Determination of Cr(Ⅵ) and Cr(Ⅲ) species in parenteral solutions using a nanostructured material packed-microcolumn and electrothermal atomic absorption spectrometry[J].Journal of Trace Elements in Medicine and Biology, 2009, 23(3):157-166. doi: 10.1016/j.jtemb.2009.03.002
[25] Lee C F, Chen B H, Huang Y L.Determining Cr(Ⅲ) and Cr(Ⅵ) in urine using a flow injection on-line sorption separation system coupled with electrothermal atomic absorption spectrometry and a UV/nano-Au/TiO2 photocatalysis reduction device[J].Talanta, 2008, 77(2):546-550. doi: 10.1016/j.talanta.2008.03.018
[26] 徐红纳, 王英滨.双波长分光光度法同时测定水样中的Cr(Ⅲ)和Cr(Ⅵ)[J].分析试验室, 2008, 27(5):34-37. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys200805009
Xu H N, Wang Y B.Simultaneous determination of chromium(Ⅲ) and chromium(Ⅵ) by dual-wavelength spectrophotometry[J].Chinese Journal of Analysis Laboratory, 2008, 27(5):34-37. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxsys200805009
[27] Hackbarth F Ⅴ, Maass D, Souza A A U, et al.Removal of hexavalent chromium from electroplating wastewaters using marine macroalga Pelvetia canaliculata as natural electron donor[J].Chemical Engineering Journal, 2016, 290:477-489. doi: 10.1016/j.cej.2016.01.070
[28] Kim J S, Choi Y R, Kim Y S, et al.Determination of hexavalent chromium (Cr(Ⅵ)) in plastics using organic-assisted alkaline extraction[J].Analytica Chimica Acta, 2011, 690(2):182-189. doi: 10.1016/j.aca.2011.01.060
[29] Abadi M D M, Chamsaz M, Arbab-Zavar M H, et al.Supramolecular dispersive liquid-liquid microextraction-based solidification of floating organic drops for speciation and spectrophotometric determination of chromium in real samples[J].Analytical Methods, 2013, 5(12):2971-2977. doi: 10.1039/c3ay00036b
[30] 孙克强, 王京力, 李浩洋, 等.离子色谱法测定玩具中三价铬和六价铬的含量[J].理化检验(化学分册), 2017, 53(9):1099-1102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lhjy-hx201709025
Sun K Q, Wang J L, Li H Y, et al.Determination of chromium(Ⅲ) and chromium(Ⅵ) in toys by ion chromatography[J].Physical Testing and Chemical Analysis (Part B:Chemical Analysis), 2017, 53(9):1099-1102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lhjy-hx201709025
[31] 巢静波, 史乃捷, 陈扬, 等.衍生液注入控制-离子色谱法同时测定环境水样中的三价铬和六价铬[J].环境化学, 2016, 35(1):67-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201601009
Chao J B, Shi N J, Chen Y, et al.Simultaneous determination of trivalent and hexavalent chromium in environmental waters by ion chromatography with derivatization reagent injection-control technique[J].Environmental Chemistry, 2016, 35(1):67-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjhx201601009
[32] Araujo-Barbosa U, Pena-Vazquez E, Barciela- Alonso M C, et al.Simultaneous determination and speciation analysis of arsenic and chromium in iron supplements used for iron-deficiency anemia treatment by HPLC-ICP-MS[J].Talanta, 2017, 170:523-529. doi: 10.1016/j.talanta.2017.04.034
[33] 古君平, 施文庄, 刘殷, 等.高效液相色谱-电感耦合等离子体质谱法同时测定烟用接装纸中三价铬与六价铬的含量[J].理化检验(化学分册), 2017, 53(1):17-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lhjy-hx201701004
Gu J P, Shi W Z, Liu Y, et al.Simultaneous determination of chromium(Ⅲ) and chromium(Ⅵ) in cigarette tipping paper by HPLC-ICP-MS[J].Physical Testing and Chemical Analysis (Part B:Chemical Analysis), 2017, 53(1):17-21. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lhjy-hx201701004
[34] 周波林.一阶导数紫外光谱法测定人血浆中地西泮的血药浓度[J].中国医药导报, 2014, 11(20):16-19. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yycyzx201420006
Zhou B L.Determination of diazepam in human plasma by first order derivative spectrophotometry[J].China Medical Herald, 2014, 11(20):16-19. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yycyzx201420006
[35] 王华建, 黎艳红, 丰伟悦, 等.反相离子对色谱-电感耦合等离子体质谱联用技术测定水中痕量Cr(Ⅲ)与Cr(Ⅵ)[J].分析化学, 2009, 37(3):443-436. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxhx200903025
Wang H J, Li Y H, Feng W Y, et al.Simultaneous determination of trace Cr(Ⅲ) and Cr(Ⅵ) in water using ion-pairing reversed phase chromatography-inductively coupled plasma mass spectrometry[J].Chinese Journal of Analytical Chemistry, 2009, 37(3):443-436. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fxhx200903025
-



 下载:
下载: