Characteristics of Carbon and Oxygen Isotope Standard Materials of Carbonates and Their Effect on Isotope Analysis and Standard Preservation
-
摘要:
碳酸盐物质碳氧同位素广泛应用于地质和气候环境研究,而标准物质是其分析的基准物质和数据比较的重要依据。不同标准物质性状存在差异,对于性状特点的了解,有助于标准的选择和使用、不均匀性的改善以及最佳实验条件的建立。本文采用X射线衍射(XRD)、光学显微镜及能谱扫描-电子显微镜(EDX-SEM)技术,从不同尺度对物源、粒径、颗粒形貌和结构,以及纯净程度等性状不同的碳酸盐碳氧同位素标准物质开展了观察和分析,并采用连续流磷酸法测定部分碳酸盐标准物质的δ13C和δ18O值,从而探讨其性状特点以及对分析和保存的影响。结果表明:天然碳酸盐标准物质普遍含有非碳酸钙成分,如少量石英;以及存在明显性状差异的颗粒,但总量较少。不同标准物质之间、标准物质颗粒之间也存在程度不同的粒径、形貌、微细结构和矿物组分等性状差异。实验测定了不同标准物质的δ13C和δ18O值,多数分析结果与推荐值吻合,其中IAEA-CO-8的δ13C和δ18O值标准偏差相比其他物质偏大,可能与该标准组成复杂且均一性较差有关,而NBS20的δ18O值标准偏差相对偏大以及7902的δ18O值与推荐值偏离较大,推测与其粉末状且组成颗粒细小的性状更容易受到空气中二氧化碳和水的影响有关。结合前人和本研究认为,在推荐使用量下天然碳酸盐标准物质性状对分析精准性造成的影响有限;而为了保证微量分析和研究的准确性,建议根据分析和研究目的,结合标准和物质的性状特点,有针对性地选择标准以及标准中的颗粒,同时粉末状且组成颗粒细小标准物质在制备和保存上需要更加注意。本研究补充和丰富了碳酸盐碳氧同位素标准物质的性状信息,有助于其更好地应用于微量碳酸盐碳氧同位素分析和研究,同时也为保存和制备提供参考。
Abstract:BACKGROUND Carbon and oxygen isotopes in carbonates are commonly used in geology and climate studies. Reference materials for these isotopes are an important basis for analysis and data comparison. Understanding the differences in the characteristics of different standard materials is helpful for the selection and use of standards, improvement of heterogeneity, and establishment of optimal experimental conditions.
OBJECTIVES To investigate the influence of the characteristics of carbon and oxygen isotope standard materials of carbonates on isotope analysis and preservation of the standards.
METHODS In this study, X-ray diffraction (XRD), optical microscopy, and energy dispersive X-ray scanning electron microscopy (EDX-SEM) were used to analyze carbonate carbon and oxygen isotope reference materials with different properties, such as provenance, particle size, particle morphology and structure, and purity. The δ13C and δ18O values of some carbonate reference materials were determined by the continuous flow phosphoric acid method to explore their characteristics and their influence on isotope analysis and standard preservation.
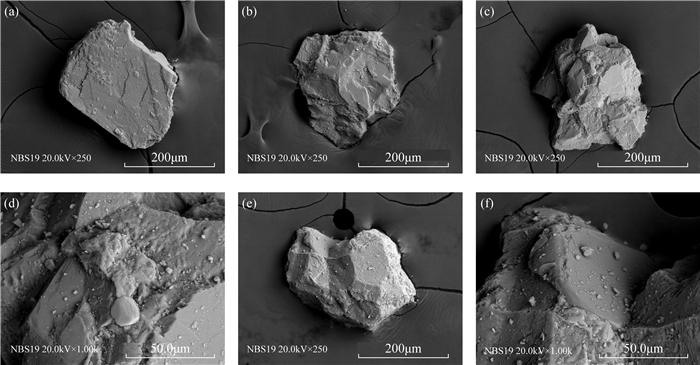
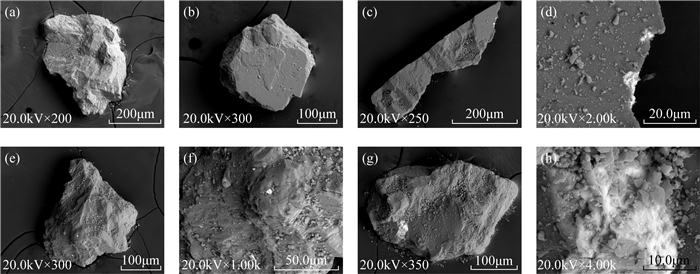
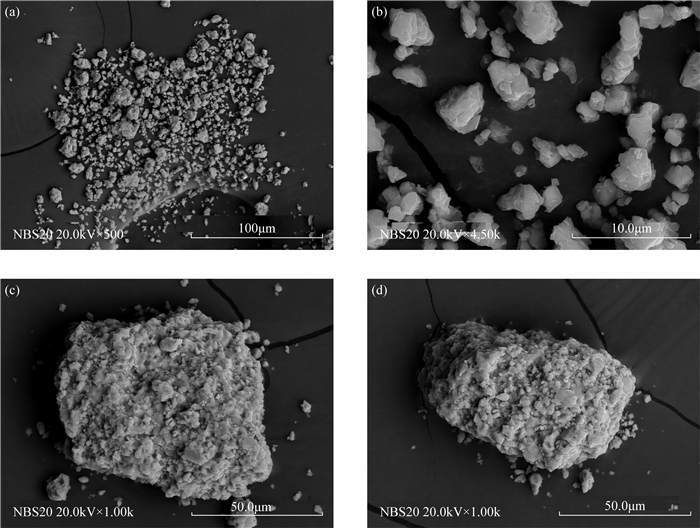
RESULTS Natural carbonate reference materials generally contain non-calcium carbonate components, such as small amounts of quartz and other particles with obvious differences in properties. There are also differences in particle size, morphology, microstructure, and mineral composition between different standard materials and between standard material particles. The δ13C and δ18O values of different standard materials were determined, and most results were consistent with the recommended values. The standard deviations of the δ13C and δ18O values of IAEA-CO-8 were larger than those of other standard materials, which is attributable to their complex composition and homogeneity. The standard deviation of the δ18O value of NBS20 was large, while the δ18O value of 7902 deviated from the recommended value. It is inferred that their powdery and fine particles are more likely to be affected by carbon dioxide and water in air.
CONCLUSIONS Based on previous research as well as the current study, it is believed that under the recommended usage amount, the properties of natural carbonate reference materials have a limited impact on the accuracy of analysis. To ensure the accuracy of microanalysis and research, while selecting the standard and the particles in the standard, it is recommended to combine the characteristics of standards and materials based on the purpose of the analysis and research. At the same time, the preparation and storage of powdery and fine-grained standard materials require more attention. This study supplements information regarding the properties of carbonate carbon and oxygen isotope standard materials, which helps in the analysis and research of trace carbonate carbon and oxygen isotopes, and provides a reference for standard preservation and preparation.
-

-
表 1 稳定同位素标准物质信息
Table 1. Standards and reference materials for stable isotopes
标准物质编号 来源 岩性 尺寸 δ13CVPDB (‰) δ18OVPDB (‰) NBS19[30] IAEA 大理岩 颗粒,粒径200~300μm 1.95 -2.2 IAEA-603[29] IAEA 大理岩 颗粒,粒径200~500μm 2.46 -2.37 NBS18[30] IAEA 碳酸岩 颗粒,粒径90~400μm -5.01 -23.0 IAEA- CO-8[18] IAEA 碳酸岩 颗粒 -5.76 -22.7 GBW04405 国家标准物质 灰岩 粉末,小于200目 0.57 -8.49 NBS20[18, 31] IAEA 灰岩 粉末 -1.06 -4.14 811 中国地质调查局成都地质调查中心(原成都地质矿产研究所) 方解石 粉末 -3.32 -10.62 7901[19] 北京大学(原北京大学地质系) 方解石 粉末 2.62 -5.50 7902[19] 北京大学(原北京大学地质系) 碳酸钙化学试剂 粉末 -6.29 -14.54 表 2 X射线衍射分析天然标准物质中的矿物组分
Table 2. Minerals components in standard materials identified by X-ray diffraction
标准物质编号 矿物成分 方解石 石英 白云石 GBW04405 有 少量 少量 TTB-1 有 少量 少量 NBS20 有 少量 - 811 有 少量 少量 7901 有 少量 少量 注:“-”表示未发现该矿物。 表 3 标准物质IAEA-CO-8、GBW04405、NBS20、811、7901、7902碳氧同位素分析结果
Table 3. Analytical results of standard and reference materials IAEA-CO-8, GBW04405, NBS20, 811, 7901 and 7902
标准物质编号 δ13CVPDB (‰) δ18OVPDB (‰) 推荐值 测定值(n≥7) 推荐值 测定值(n≥7) IAEA-CO-8 -5.76±0.03 -5.77±0.05 -22.7±0.2 -22.6±0.1 GBW04405 0.57±0.03 0.57±0.02 -8.49±0.14 -8.51±0.05 NBS20 1.06±0.02 1.05±0.03 -4.14±0.03 -4.13±0.08 811 -3.32 -3.34±0.04 -10.62 -10.64±0.06 7901 2.62 2.57±0.03 -5.50 -5.49±0.03 7902 -6.29 -6.28±0.02 -14.54 -14.05±0.04 -
[1] 郑永飞, 陈江峰. 稳定同位素地球化学[M]. 北京: 科学出版社, 2000.
Zheng Y F, Chen J F. Stable isotope geochemistry[M]. Beijing: Science Press, 2000.
[2] 严兆彬, 郭福生, 潘家永, 等. 碳酸盐岩C, O, Sr同位素组成在古气候、古海洋环境研究中的应用[J]. 地质找矿论丛, 2005, 20(1): 53-56. doi: 10.3969/j.issn.1001-1412.2005.01.010
Yan Z B, Guo F S, Pan J Y, et al. Application of C, O and Sr isotope composition of carbonates in the research of paleoclimate and paleooceanic environment[J]. Contributions to Geology and Mineral Resources Research, 2005, 20(1): 53-56. doi: 10.3969/j.issn.1001-1412.2005.01.010
[3] Scobar J E, Curtis J H, Brenner M, et al. Isotope mea-surements of single ostracod values and gastropod shells for climates reconstruction: Evaluation of within-sample variability and determination of optimum sample size[J]. Journal of Palealimnology, 2010, 43: 921. doi: 10.1007/s10933-009-9377-9
[4] Charlier B L A, Ginibre C, Morgan D, et al. Methods for the microsampling and high-precision analysis of strontium and rubidium isotopes at single crystal scale for petrological and geochronological applications[J]. Chemical Geology, 2006, 232(3): 113-114. http://www.sciencedirect.com/science/article/pii/S0009254106001367
[5] 邓文峰, 韦刚健, 李献华. 不纯碳酸盐碳氧同位素组成的在线分析[J]. 地球化学, 2005, 34(5): 495-500. doi: 10.3321/j.issn:0379-1726.2005.05.007
Deng W F, Wei G J, Li X H. Online analysis of carbon and oxygen isotopic compositions of impure carbonate[J]. Geochimica, 2005, 34(5): 495-500. doi: 10.3321/j.issn:0379-1726.2005.05.007
[6] 陶成, 把立强, 李广友, 等. GasBench-IRMS在碳酸盐岩δ13C和δ18O在线连续分析中的应用[J]. 岩矿测试, 2006, 25(4): 334-336. doi: 10.3969/j.issn.0254-5357.2006.04.009 http://www.ykcs.ac.cn/article/id/ykcs_200604111
Tao C, Ba L Q, Li G Y, et al. Application of GasBench-IRMS in on-line continuous measurement of δ13C and δ18O in carbonate rock samples[J]. Rock and Mineral Analysis, 2006, 25(4): 334-336. doi: 10.3969/j.issn.0254-5357.2006.04.009 http://www.ykcs.ac.cn/article/id/ykcs_200604111
[7] 杜广鹏, 王旭, 张福松. GasBenchⅡ顶空瓶内空气背景对 < 100μg碳酸盐中碳氧同位素在线测定的影响及校正方法初探[J]. 岩矿测试, 2010, 29(6): 631-638. doi: 10.3969/j.issn.0254-5357.2010.06.001
Du G P, Wang X, Zhang F S. Influence of the air background in GasBenchⅡ vials on the measurements of C and O isotopes in carbonates and a preliminary study on blank correction strategy[J]. Rock and Mineral Analysis, 2010, 29(6): 631-638. doi: 10.3969/j.issn.0254-5357.2010.06.001
[8] 朱园园, 邱海鸥, 杜永, 等. 应用GasBenchⅡ-IRMS优化碳氧同位素分析方法[J]. 岩矿测试, 2014, 33(6): 789-794. http://www.ykcs.ac.cn/article/id/4ac7f375-185a-4aa0-a0a9-7ac95ae010cf
Zhu Y Y, Qiu H O, Du Y, et al. Evaluation and optimization of carbon and oxygen isotopes experimental conditions determinated by GasBenchⅡ-IRMS method[J]. Rock and Mineral Analysis, 2014, 33(6): 789-794. http://www.ykcs.ac.cn/article/id/4ac7f375-185a-4aa0-a0a9-7ac95ae010cf
[9] 梁翠翠, 尹希杰, 徐勇航, 等. GasBenchⅡ-IRMS测定微量碳酸盐中碳氧同位素比值方法研究[J]. 同位素, 2015, 28(1): 41-47. https://www.cnki.com.cn/Article/CJFDTOTAL-TWSZ201501008.htm
Liang C C, Yin X J, Xu Y H, et al. Analytical method for carbon and oxygen isotope of small carbonate samples with the GasBenchⅡ-IRMS devices[J]. Journal of Isotopes, 2015, 28(1): 41-47. https://www.cnki.com.cn/Article/CJFDTOTAL-TWSZ201501008.htm
[10] Zha X P, Zhao Y Y, Zheng Y F. An online method combining a GasBenchⅡ with continuous flow isotope ratio mass spectrometry to determine the content and isotopic compositions of minor amounts of carbonate in silicate rocks[J]. Rapid Communications in Mass Spectrometry, 2010, 24(15): 2217-2226. doi: 10.1002/rcm.4632
[11] Breitenbach S F M, Bernasconi S M. Carbon and oxygen isotope analysis of small carbonate samples (20 to 100μg) with a GasBenchⅡ preparation device[J]. Rapid Communications in Mass Spectrometry, 2011, 25(13): 1910-1914. doi: 10.1002/rcm.5052
[12] 丁悌平. 稳定同位素测试技术与参考物质研究现状及发展趋势[J]. 岩矿测试, 2002, 21(4): 291-300. doi: 10.3969/j.issn.0254-5357.2002.04.011 http://www.ykcs.ac.cn/article/id/ykcs_20020477
Ding T P. Present status and prospect of analytical techniques and reference materials for stable isotopes[J]. Rock and Mineral Analysis, 2002, 21(4): 291-300. doi: 10.3969/j.issn.0254-5357.2002.04.011 http://www.ykcs.ac.cn/article/id/ykcs_20020477
[13] Tang G Q, Li X H, Li Q L, et al. A new Chinese national reference material (GBW04481) for calcite oxygen and carbon isotopic microanalysis[J]. Surface and Interface Analysis, 2019, 52(5): 190-196. http://onlinelibrary.wiley.com/doi/10.1002/sia.6712
[14] Ishimura T, Tsunogai U, Gamo T. Stable carbon and oxygen isotopic determination of sub-microgram quantities of CaCO3 to analyze individual foraminiferal shells[J]. Rapid Communications in Mass Spectrometry, 2004, 18(23): 2883-2888. doi: 10.1002/rcm.1701
[15] Kimoto K, Ishimura T, Tsunogai U, et al. The living triserial planktic foraminifer Gallitelliavivans (Cushman): Distribution, stable isotopes, and paleoecological implications[J]. Marine Micropaleon-tology, 2009, 71(1): 71-79. http://www.sciencedirect.com/science/article/pii/S0377839809000073
[16] Ishimura T, Tsunogai U, Hasegawa S, et al. Variation in stable carbon and oxygen isotopes of individual benthic for aminifera: Tracers for quantifying the magnitude of isotopic disequilibrium[J]. Biogeoences Discussions, 2012, 9(5): 6191-6218. http://www.oalib.com/paper/1375011
[17] Zha X P, Gong B, Zheng Y F, et al. Precise carbon isotopic ratio analyses of micro amounts of carbonate and non-carbonate in basalt using continuous-flow isotope ratio mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2018, 32(1): 48-56. doi: 10.1002/rcm.8008
[18] Brand W A, Coplen T B, Vogl J, et al. Assessment of international reference materials for isotope-ratio analysis (IUPAC technical report)[J]. Pure and Applied Chemistry, 2014, 86(3): 425-467. doi: 10.1515/pac-2013-1023
[19] 郑淑蕙, 郑斯成, 莫志超. 稳定同位素地球化学分析[M]. 北京: 北京大学出版社, 1986: 402.
Zheng S H, Zheng S C, Mo Z C. Stable isotope geochemical analysis[M]. Beijing: Peking University Press, 1986: 402.
[20] Lin Y, Feng L, Hao J, et al. Sintering nano-crystalline calcite: A new method of synthesizing homogeneous reference materials for SIMS analysis[J]. Journal of Analytical Atomic Spectrometry, 2014, 29(9): 1686-1691. doi: 10.1039/C4JA00136B
[21] Helie J F, Hillaire-Marcel C, Groening M. Suitability of IAEA-603 as a replacement to NBS19 for small sample analysis[C]//Report to IAEA-Terrestrial Environment Laboratory. Montreal: GEOTOP-UQAM, 2013: 1-11.
[22] Assonov S, Groening M, Fajgelj A, et al. Preparation and characterization of IAEA-603, a new primary reference material aimed at the VPDB scale realisation for δ13C and δ18O determination[J]. Rapid Communications in Mass Spectrometry, 2020, 34(20): e8867. http://onlinelibrary.wiley.com/doi/10.1002/rcm.8867
[23] Helie J F, Hillaire-Marcel C. Designing working standards for stable H, C and O isotope measurements in CO2 and H2O[J]. Rapid Communications in Mass Spectrometry, 2021, 35(5): e9008. http://onlinelibrary.wiley.com/doi/10.1002/rcm.9008
[24] Assonov S, Gröning M, Fajgelj A. IAEA stable isotope reference materials: Addressing the needs of atmospheric greenhouse gas monitoring[C]//The 18th WMO/IAEA meeting on carbon dioxide, other greenhouse gases and related tracers measurement techniques (GGMT-2015). California: GAW Report No. 229: 76-80.
[25] Ishimura T, Tsunogai U, Nakagawa F. Grain-scale hete-rogeneities in the stable carbon and oxygen isotopic compositions of the international standard calcite materials (NBS19, NBS18, IAEA-CO-1, and IAEA-CO-8)[J]. Rapid Communications in Mass Spectrometry, 2008, 22(12): 1925-1932. doi: 10.1002/rcm.3571
[26] Crowley S F. Mineralogical and chemical composition of international carbon and oxygen isotope calibration material NBS19, andreference materials NBS18, IAEA-CO-1 and IAEA-CO-8[J]. Geostandards and Geoanalytical Research, 2010, 34(2): 193-206. doi: 10.1111/j.1751-908X.2010.00037.x
[27] Nishida K, Ishimura T. Grain-scale stable carbon and oxygen isotopic variations of the international reference calcite, IAEA-603[J]. Rapid Communications in Mass Spectrometry, 2017, 31(22): 1875-1880. doi: 10.1002/rcm.7966
[28] 杨会, 唐伟, 吴夏, 等. KielⅣ-IRMS双路在线分析微量碳酸盐的碳氧同位素[J]. 岩矿测试, 2014, 33(4): 480-485. doi: 10.3969/j.issn.0254-5357.2014.04.004 http://www.ykcs.ac.cn/article/id/ccc63571-9e6a-441a-bcd1-43284a2c2fb6
Yang H, Tang W, Wu X, et al. Carbon and oxygen isotope analysis of trace carbonate by Kiel Ⅳ-IRMS using on-line dual technique[J]. Rock and Mineral Analysis, 2014, 33(4): 480-485. doi: 10.3969/j.issn.0254-5357.2014.04.004 http://www.ykcs.ac.cn/article/id/ccc63571-9e6a-441a-bcd1-43284a2c2fb6
[29] Reference sheet for IAEA-603[R]. Vienna: International Atomic Energy Agency, 2016.
[30] Friedman I, O'Neil J, Cebula G. Two new carbonate stable isotope standards[J]. Geostandard Newsletter, 1982, 6(1): 11-12. doi: 10.1111/j.1751-908X.1982.tb00340.x
[31] Craig H. Isotopic standards for carbon and oxygen and correction factors for mass spectrometric analysis of carbon dioxide[J]. Geochimica Et Cosmochimica Acta, 1957, 12(1-2): 133-149. doi: 10.1016/0016-7037(57)90024-8
[32] 王凤玉, 胡志中, 杜谷. X射线衍射法在有机药物研究中的运用[J]. 资源开发与市场, 2014, 30(9): 1030-1031. doi: 10.3969/j.issn.1005-8141.2014.09.002
Wang F Y, Hu Z Z, Du G. Progresses on X-ray diffraction analysis in organic drug research[J]. Resource Development and Market, 2014, 30(9): 1030-1031. doi: 10.3969/j.issn.1005-8141.2014.09.002
[33] 王坤阳, 杜谷, 杨玉杰, 等. 应用扫描电镜与X射线能谱仪研究黔北黑色页岩储层孔隙及矿物特征[J]. 岩矿测试, 2014, 33(5): 634-639. doi: 10.3969/j.issn.0254-5357.2014.05.004 http://www.ykcs.ac.cn/article/id/b80c341c-6297-43b0-a61b-daaaddfa9611
Wang K Y, Du G, Yang Y J, et al. Characteristics study of reservoirs pores and mineral compositions for black shale, northern Guizhou, by using SEM and X-ray EDS[J]. Rock and Mineral Analysis, 2014, 33(5): 634-639. doi: 10.3969/j.issn.0254-5357.2014.05.004 http://www.ykcs.ac.cn/article/id/b80c341c-6297-43b0-a61b-daaaddfa9611
[34] 张琳, 刘福亮, 贾艳琨, 等. 稳定同位素分析数据标准化校准方法的讨论[J]. 环境化学, 2011, 30(3): 727-728. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX201103032.htm
Zhang L, Liu F L, Jia Y K, et al. Discussion on standardized calibration methods for stable isotope analysis date[J]. Environmental Chemistry, 2011, 30(3): 727-728. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX201103032.htm
[35] Paul D S G, Fórizs I. Normalization of measured stable isotopic com positions to isotope reference scales-A review[J]. Rapid Communications in Mass Spectrometry, 2007, 21(18): 3006-3014. doi: 10.1002/rcm.3185
[36] 于吉顺, 雷新荣, 张锦化, 等. 矿物X射线粉晶鉴定手册(图谱)[M]. 武汉: 华中科技大学出版社, 2011.
Yu J S, Lei X R, Zhang J H, et al. Mineral X-ray powder identification manual[M]. Wuhan: Huazhong University of Science and Technology Press, 2011.
[37] 何道清. 激光微取样稳定同位素分析新技术[J]. 石油仪器, 1997, 11(5): 41-44. https://www.cnki.com.cn/Article/CJFDTOTAL-SYYQ199705007.htm
He D Q. The new technique of laser microsampling of isotopic analysis[J]. Petroleum Instruments, 1997, 11(5): 41-44. https://www.cnki.com.cn/Article/CJFDTOTAL-SYYQ199705007.htm
[38] Ball J D, Crowley S F, Steele D F. Carbon and oxygen isotope ratio analysis of small carbonate samples by conventional phosphoric acid digestion: Sample preparation and calibration[J]. Rapid Communications in Mass Spectrometry, 1996, 10(8): 987-995. doi: 10.1002/(SICI)1097-0231(19960610)10:8<987::AID-RCM537>3.0.CO;2-D
[39] Barber D J, Wenk H R. Microstructure in carbonates from the Alnø and Fen carbonatites[J]. Contributions to Mineralogy and Petrology, 1984, 88(3): 233-245. doi: 10.1007/BF00380168
[40] Hornig-Kjarsgaard I. Rare earth elements in Sövitic carbonatites and their mineral phases[J]. Journal of Petrology, 1998, 39(11-12): 2105-2121. doi: 10.1093/petroj/39.11-12.2105
[41] 李文军, 张青莲. 三种国际碳氧同位素参考物质的比较测定[J]. 化学通报, 1987(11): 33-34. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB198711006.htm
Li W J, Zhang Q L. A comparative study on reference materials of carbon and oxygen isotopes[J]. Chemistry, 1987(11): 33-34. https://www.cnki.com.cn/Article/CJFDTOTAL-HXTB198711006.htm
[42] Gonfiantini R, Stichler W, Rozanski K. Standards and intercomparison materials distributed by the international atomic energy agency for stable isotope measurement[R]//Referenceand intercomparison materials for stable isotopes of light elements. Vienna: International Atomic Energy Agency (IAEA-TECDOC-825), 1995: 13-29. http://www.researchgate.net/publication/311570392_Standards_and_intercomparison_materials_distributed_by_the_International_Atomic_Energy_Agency_for_stable_isotope_measurements
[43] Liu X, Deng W F, Wei G J. Carbon and oxygen isotopic analyses of calcite in calcite-dolomite mixtures: Optimization of selective acid extraction[J]. Rapid Communications in Mass Spectrometry, 2019, 33(5): 411-418. doi: 10.1002/rcm.8365
[44] Assonov S, Fajgelj A, Helie J F, et al. Characterisation of new reference materials IAEA-610, IAEA-611 and IAEA-612 aimed at the VPDB δ13C scale realisation with small uncertainty[J]. Rapid Communications in Mass Spectrometry, 2020, 35(7): e9014. http://onlinelibrary.wiley.com/doi/pdf/10.1002/rcm.9014
[45] Assonov S. Summary and recommendations from the international atomic energy agency technical meeting on the development of stable isotope reference products (21-25 November 2016)[J]. Rapid Communications in Mass Spectrometry, 2018, 32(10): 827-830. doi: 10.1002/rcm.8102
-




 下载:
下载: