The Application of EPMA in the Textural Characterization of Cryptomelane in the Xialei Manganese Deposit, Southwest Guangxi
-
摘要:
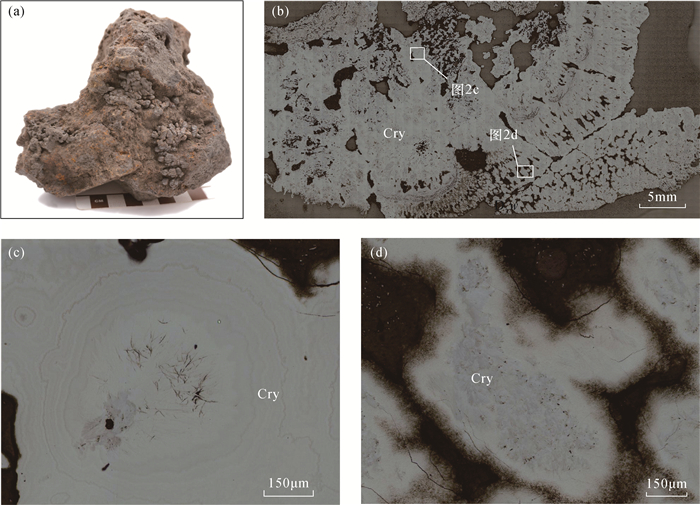
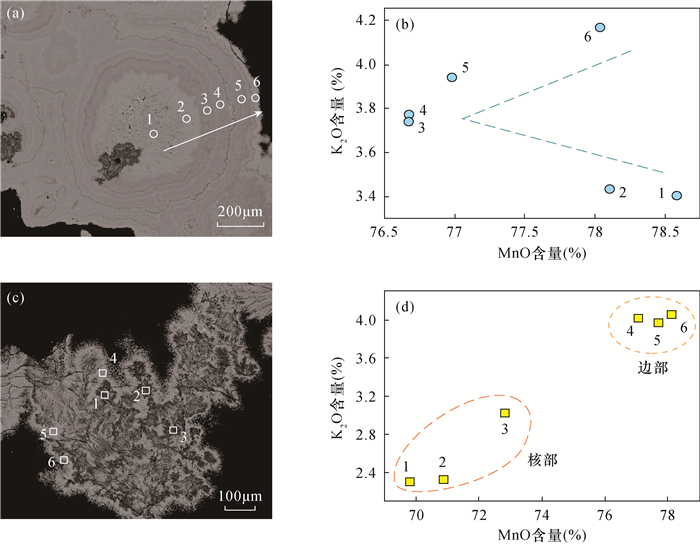
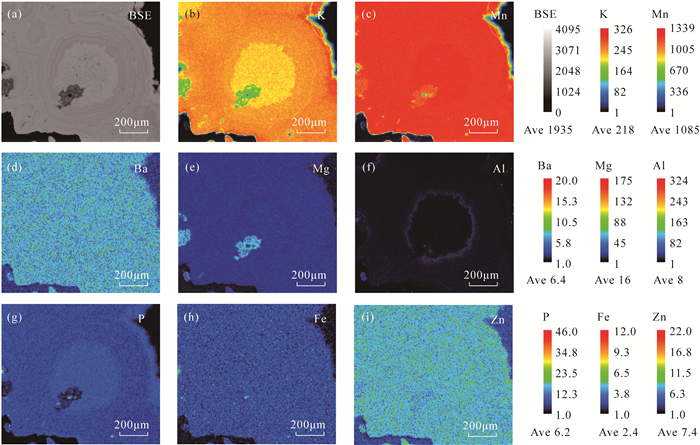
八面体分子筛(OMS-2)具有2×2孔道结构,在离子交换、催化剂、能源和环境等方面具有非常重要的应用价值,然而天然OMS-2矿物材料——锰钾矿在典型结构的成分精细表征和成因研究等方面仍然缺乏。环带和核-边结构在锰氧化物矿物的结构中非常具有代表性,明确其矿物种属、探索其成分特征对于探究其成因、开拓锰氧化物的应用具有重要意义。广西锰矿资源储量占中国锰矿资源储量的23%,其中位于桂西南的下雷锰矿是中国最早发现的超大型锰矿床,氧化锰矿石平均品位30%左右。本研究主要利用电子探针(EPMA)定量分析和面扫描等手段针对下雷锰矿中的环带和核-边结构开展研究。结果表明:下雷锰矿产出具有环带结构和核-边结构的锰钾矿,具有环带结构的锰钾矿元素强度按平均强度排序从强至弱依次为Mn、K、Mg、Al、Zn、Ba、P、Fe,具有核-边结构的锰钾矿元素强度按平均强度排序从强至弱依次为Mn、K、Ca、Mg、Zn、Ba、P、Fe,这些元素从内到外的变化反映了其氧化环境的变化。从中间到边部钾含量逐渐升高(质量分数为2.31%~4.17%,单位分子式中原子数为0.38~0.62),暗示了氧化过程中钾的富集,也反映了锰氧化物逐渐趋于最稳定状态的过程。钾离子和锰离子趋势的变化是导致环带和核-边结构形成的直接原因,氧化环境的变化和锰氧化物逐渐趋于最稳定状态的趋势可能是锰钾矿环带和核-边结构形成的根本原因。
Abstract:BACKGROUND OMS-2 has shown great significance in ion exchange, catalyst, energy and environment, but the research of natural OMS-2 mineral material cryptomelane is still lacking in the detailed characterization of the composition, structure and genetic research. Annulus and core-rim structures are representative in the structure of manganese oxide minerals. It is of great significance to clarify the mineral species and explore its composition characteristics, its origin and develop the application of manganese oxides. The Mn ore resource of Guangxi account for 23% of the manganese ore resource reserves in China. The Xialei Mn deposit located in Southwest Guangxi is the earliest super-large Mn deposit discovered in China, with an average grade of about 30% for the manganese oxide ore.
OBJECTIVES To explore the annulus and core-rim texture and its composition of cryptomelane in the Xialei Mn deposit.
METHODS Quantitative analysis and element mapping of EPMA and microscopy were carried out.
RESULTS The element intensity of the cryptomelane with annulus texture was Mn, K, Mg, Al, Zn, Ba, P, Fe in order of average from strong to weak. The average element intensity of the cryptomelane with core-rim texture was Mn, K, Ca, Mg, Zn, Ba, P, Fe from strong to weak. The K content gradually increased (2.31%-4.17%, 0.38-0.62 atoms per formula unit) from the middle to the rim of the cryptomelane, indicating K enrichment during oxidization and the stable status of Mn oxides.
CONCLUSIONS The change in the trend of K and Mn ions is the direct cause of the formation of the annulus and core-rim texture, reflecting the changes of oxidation environment. Potassium content gradually increases from the inner to outer zone, which may indicate the enrichment of potassium during the oxidation process, and also reflects manganese oxide gradually tending to the most stable state.
-

-
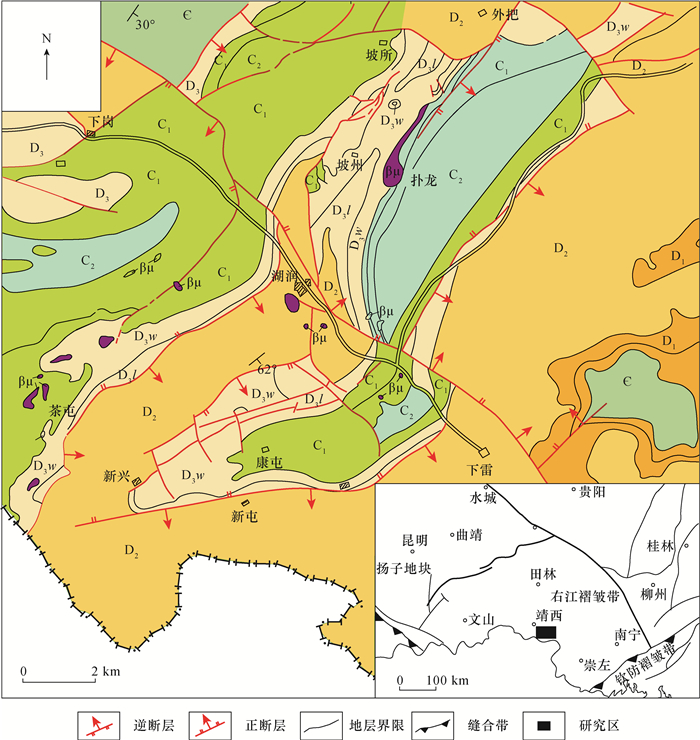
图 1 桂西南下雷锰矿矿区地质图(据赵立群等[36])
Figure 1.
表 1 广西下雷锰矿锰钾矿电子探针定量分析数据
Table 1. Chemical composition of the cryptomelane from the Xialei manganese deposit measured by EPMA
测点编号 各成分含量(%) Al2O3 SiO2 CaO P2O5 BaO ZrO2 PbO TiO2 FeO Cr2O3 NiO CuO Na2O MgO ZnO MnO2 K2O SrO SO3 总计 环带1-1 0.04 0.08 0.64 0.47 0.02 0.02 0.02 0.04 0.15 0.05 0.00 0.08 0.12 0.06 0.14 96.04 3.41 0.16 0.01 101.52 环带1-2 0.02 0.06 0.67 0.46 0.00 0.00 0.05 0.00 0.05 0.00 0.00 0.06 0.05 0.03 0.09 95.46 3.44 0.15 0.01 100.60 环带1-3 0.15 0.12 0.66 0.26 0.00 0.02 0.00 0.09 0.04 0.00 0.00 0.06 0.02 0.06 0.10 93.71 3.74 0.13 0.17 99.34 环带1-4 0.16 0.10 0.67 0.24 0.00 0.01 0.02 0.05 0.06 0.02 0.00 0.11 0.06 0.09 0.12 93.72 3.77 0.21 0.13 99.53 环带1-5 0.13 0.07 0.63 0.28 0.00 0.06 0.00 0.00 0.04 0.00 0.00 0.06 0.06 0.07 0.10 94.09 3.94 0.22 0.04 99.78 环带1-6 0.04 0.06 0.50 0.57 0.00 0.00 0.05 0.02 0.01 0.06 0.05 0.08 0.11 0.03 0.11 95.62 4.17 0.12 0.02 101.62 环带2-1 0.01 0.02 0.03 0.08 0.11 0.00 0.00 0.00 0.04 0.06 0.14 0.01 0.02 0.03 0.08 94.76 3.38 0.11 0.02 98.71 环带2-2 0.00 0.05 0.00 0.00 0.13 0.02 0.05 0.02 0.04 0.03 0.14 0.00 0.05 0.00 0.00 94.54 3.33 0.09 0.00 98.39 环带2-3 0.00 0.00 0.00 0.06 0.11 0.04 0.13 0.00 0.08 0.05 0.19 0.00 0.00 0.00 0.06 91.84 3.70 0.13 0.08 96.41 环带2-4 0.01 0.00 0.00 0.01 0.12 0.05 0.17 0.00 0.08 0.06 0.19 0.01 0.00 0.00 0.01 92.04 3.70 0.18 0.07 96.66 环带2-5 0.00 0.01 0.00 0.00 0.09 0.07 0.16 0.08 0.07 0.08 0.13 0.00 0.01 0.00 0.00 91.85 3.73 0.14 0.11 96.50 环带2-6 0.01 0.00 0.00 0.00 0.06 0.03 0.07 0.01 0.03 0.06 0.17 0.01 0.00 0.00 0.00 93.13 3.87 0.15 0.05 97.64 核1-1 0.12 0.04 1.45 0.00 0.15 0.00 0.01 0.00 0.06 0.00 0.00 0.00 0.09 1.98 0.00 85.40 2.31 0.07 0.01 91.68 核1-2 0.11 0.00 1.37 0.00 0.14 0.00 0.00 0.00 0.12 0.02 0.00 0.01 0.06 1.96 0.02 86.61 2.33 0.04 0.01 92.81 核1-3 0.17 0.04 1.21 0.15 0.08 0.00 0.02 0.12 0.04 0.02 0.00 0.07 0.06 1.02 0.14 89.00 3.02 0.17 0.02 95.33 边1-4 0.15 0.06 0.47 0.72 0.02 0.01 0.02 0.00 0.05 0.02 0.00 0.00 0.09 0.00 0.18 94.20 4.02 0.19 0.01 100.21 边1-5 0.14 0.04 0.64 0.66 0.02 0.01 0.00 0.03 0.12 0.00 0.00 0.00 0.06 0.05 0.00 94.94 3.97 0.11 0.02 100.81 边1-6 0.13 0.06 0.46 0.72 0.00 0.02 0.03 0.07 0.11 0.04 0.03 0.03 0.09 0.04 0.16 95.49 4.07 0.18 0.00 101.72 核2-1 0.04 0.10 1.13 0.16 0.07 0.00 0.04 0.00 0.16 0.00 0.09 0.01 0.09 1.16 0.13 88.21 2.97 0.10 0.03 92.98 核2-2 0.04 0.07 1.11 0.30 0.00 0.00 0.04 0.04 0.14 0.00 0.13 0.05 0.14 0.46 0.12 91.09 3.39 0.16 0.01 95.75 核2-3 0.04 0.07 1.22 0.23 0.06 0.00 0.00 0.02 0.19 0.03 0.10 0.00 0.06 0.85 0.25 89.01 3.12 0.13 0.00 93.74 边2-4 0.11 0.07 0.43 0.53 0.00 0.01 0.00 0.00 0.04 0.01 0.05 0.04 0.00 0.03 0.13 94.20 3.84 0.12 0.01 98.47 边2-5 0.12 0.06 0.45 0.74 0.00 0.00 0.02 0.00 0.08 0.01 0.04 0.02 0.05 0.03 0.27 94.37 3.77 0.12 0.01 98.79 边2-6 0.10 0.05 0.44 0.59 0.01 0.00 0.01 0.03 0.12 0.06 0.00 0.01 0.08 0.01 0.11 94.36 3.86 0.11 0.02 98.78 最大值 0.37 0.12 1.45 0.74 0.15 0.06 0.05 0.12 0.19 0.07 0.17 0.11 0.14 1.98 0.27 96.04 4.17 0.22 0.17 - 最小值 0.02 0.00 0.43 0.00 0.00 0.00 0.00 0.00 0.01 0.00 0.00 0.00 0.00 0.00 0.00 85.40 2.31 0.04 0.00 - 平均值 0.11 0.06 0.78 0.39 0.03 0.01 0.01 0.03 0.10 0.02 0.05 0.03 0.07 0.38 0.13 92.39 3.48 0.13 0.03 - 单位分子中阳离子数 测点编号 Al2O3 SiO2 CaO P2O5 BaO ZrO2 PbO TiO2 FeO Cr2O3 NiO CuO Na2O MgO ZnO MnO2 K2O SrO SO3 环带1-1 0.005 0.009 0.080 0.046 0.001 0.001 - 0.003 0.014 0.005 - 0.011 0.009 0.003 0.012 7.718 0.506 0.010 0.001 环带1-2 0.003 0.007 0.084 0.046 - - 0.002 - 0.005 - - 0.009 0.004 0.002 0.008 7.739 0.514 0.010 0.001 环带1-3 0.022 0.015 0.084 0.026 - 0.001 - 0.008 0.004 - - 0.008 0.002 0.003 0.009 7.700 0.568 0.009 0.015 环带1-4 0.023 0.012 0.085 0.024 - - 0.001 0.004 0.006 0.002 - 0.016 0.004 0.005 0.010 7.697 0.572 0.014 0.012 环带1-5 0.019 0.008 0.080 0.028 - 0.003 - - 0.003 - - 0.009 0.004 0.004 0.009 7.718 0.597 0.015 0.004 环带1-6 0.006 0.007 0.062 0.056 - - 0.002 0.002 0.001 0.005 0.005 0.011 0.008 0.002 0.009 7.697 0.620 0.008 0.002 环带2-1 0.006 0.004 0.075 0.047 0.001 0.001 0.001 0.007 0.011 - - - 0.003 0.003 0.012 7.734 0.510 0.007 0.001 环带2-2 0.008 0.002 0.085 0.048 - 0.003 - - 0.013 0.002 0.004 0.003 0.003 0.002 0.012 7.735 0.503 0.006 - 环带2-3 0.053 0.013 0.090 0.039 - - - 0.006 0.011 0.004 0.012 - 0.006 0.003 0.017 7.658 0.570 0.009 0.007 环带2-4 0.030 0.012 0.081 0.030 0.001 - - 0.001 0.012 0.005 0.016 - 0.006 0.003 0.017 7.692 0.571 0.012 0.007 环带2-5 0.014 0.007 0.085 0.032 - 0.001 - - 0.009 0.007 0.016 0.011 0.005 0.004 0.012 7.695 0.578 0.010 0.010 环带2-6 0.009 0.008 0.090 0.030 - - - - 0.006 0.003 0.007 0.002 0.002 0.004 0.015 7.719 0.593 0.010 0.005 核1-1 0.018 0.005 0.199 - 0.008 - - - 0.007 - - - 0.006 0.102 - 7.580 0.379 0.005 0.001 核1-2 0.017 - 0.187 - 0.007 - - - 0.012 0.002 - 0.001 0.004 0.102 0.002 7.594 0.377 0.003 0.001 核1-3 0.024 0.005 0.160 0.015 0.004 - 0.001 0.011 0.004 0.002 - 0.010 0.004 0.055 0.012 7.623 0.478 0.012 0.001 边1-4 0.021 0.007 0.059 0.072 0.001 - 0.001 - 0.005 0.002 - - 0.007 - 0.016 7.680 0.606 0.013 0.001 边1-5 0.019 0.005 0.080 0.066 0.001 0.001 - 0.002 0.011 - - - 0.004 0.003 - 7.687 0.594 0.008 0.002 边1-6 0.017 0.007 0.058 0.071 - 0.001 0.001 0.006 0.011 0.004 0.003 0.004 0.006 0.002 0.014 7.671 0.603 0.012 - 核2-1 0.006 0.012 0.151 0.017 0.003 - 0.001 - 0.016 - 0.009 0.002 0.006 0.061 0.012 7.625 0.474 0.007 0.003 核2-2 0.005 0.009 0.145 0.031 - - 0.001 0.003 0.014 - 0.013 0.007 0.010 0.025 0.010 7.661 0.527 0.011 0.001 核2-3 0.006 0.008 0.162 0.024 0.003 - - 0.002 0.019 0.003 0.010 - 0.004 0.045 0.023 7.635 0.494 0.009 - 边2-4 0.015 0.008 0.055 0.054 - - - - 0.004 0.001 0.005 0.006 - 0.002 0.011 7.718 0.581 0.008 0.001 边2-5 0.017 0.007 0.057 0.074 - - 0.001 - 0.007 0.001 0.004 0.003 0.004 0.001 0.024 7.686 0.567 0.008 0.001 边2-6 0.014 0.005 0.056 0.059 - - - 0.002 0.011 0.006 - 0.001 0.006 0.001 0.010 7.706 0.583 0.008 0.002 最大值 0.053 0.015 0.199 0.074 0.008 0.003 0.002 0.011 0.019 0.007 0.016 0.016 0.010 0.102 0.024 7.739 0.620 0.015 0.015 最小值 0.003 - 0.055 - - - - - 0.001 - - - - - - 7.580 0.377 0.003 - 平均值 0.016 0.007 0.101 0.039 0.001 - - 0.003 0.010 0.002 0.005 0.004 0.005 0.020 0.012 7.679 0.532 0.009 0.003 注:环带1-1至1-6指对下雷锰矿具有环带结构的锰钾矿中从中间依次向边部进行电子探针分析,分析位置见图 3a;环带2-1至2-6用于数据验证(选取另一采自下雷锰矿具有环带结构的锰钾矿中从中间依次向边部进行电子探针分析)。核-边1-6指对下雷锰矿具有核-边结构的锰钾矿进行电子探针分析,核1-1至1-3为核部,边1-4至1-6为边部,分析位置见图 3c;核-边2-1至2-6用于数据验证(选取另一采自下雷锰矿具有核边结构的锰钾矿,核2-1至2-3为核部电子探针数据,边2-4到6为边部电子探针数据)。
表中变价元素Mn以+4价计算。0.00表示低于检测限。 -
[1] Post J E, Mckeown D A, Heaney P J. Raman spectroscopy study of manganese oxides: Tunnel structures[J]. American Mineralogist, 2020, 105: 1175-1190. doi: 10.2138/am-2020-7390
[2] 吕国诚, 廖立兵, 李雨鑫, 等. 快速发展的中国矿物材料研究——十年进展(2011~2020年)[J]. 矿物岩石地球化学通报, 2020, 39(4): 714-725. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH202004006.htm
Lyu G C, Liao L B, Li Y X, et al. Rapid development of the mineral materials research in China—Progress in the past decade (2011—2020)[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2020, 39(4): 714-725. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH202004006.htm
[3] 何宏平, 朱建喜, 陈锰, 等. 矿物结构与矿物物理研究进展综述(2011~2020年)[J]. 矿物岩石地球化学通报, 2020, 39(4): 697-713. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH202004004.htm
He H P, Zhu J X, Chen M, et al. Progresses in researches on mineral structure and mineral physics (2011—2020)[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2020, 39(4): 697-713. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH202004004.htm
[4] Lu A H, Li Y, Liu F F, et al. The photogeochemical cycle of Mn oxides on earth's surface[J]. Mineralogical Magazine, 2021, 85(1): 1-57. doi: 10.1180/mgm.2021.12
[5] 鲁安怀, 李艳, 丁竑瑞, 等. 地表"矿物膜": 地球"新圈层"[J]. 岩石学报, 2019, 35(1): 119-128. https://www.cnki.com.cn/Article/CJFDTOTAL-YSXB201901009.htm
Lu A H, Li Y, Ding H R, et al. "Mineral membrane" of the surface: "New sphere" of the Earth[J]. Acta Petrologica Sinica, 2019, 35(1): 119-128. https://www.cnki.com.cn/Article/CJFDTOTAL-YSXB201901009.htm
[6] 鲁安怀, 李艳, 丁竑瑞, 等. 天然矿物光电效应: 矿物非经典光合作用[J]. 地学前缘, 2020, 27(5): 179-194. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202005018.htm
Lu A H, Li Y, Ding H R, et al. Natural mineral photoelectric effect: Non-classical mineral photosynthesis[J]. Earth Science Frontiers, 2020, 27(5): 179-194. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202005018.htm
[7] Ma D, Wu J, Yang P, et al. Coupled manganese redox cycling and organic carbon degradation on mineral surfaces[J]. Environmental Science and Technology, 2020, 54(14): 8801-8810. doi: 10.1021/acs.est.0c02065
[8] Zhang Q, Didier C, Pang W K, et al. Structural insight into layer gliding and lattice distortion in layered manganese oxide electrodes for potassium-ion batteries[J]. Advanced Energy Materials, 2019: 1900568.
[9] 李灵桐, 臧晓蓓, 曹宁. 锌锰二次电池研究进展[J]. 材料导报, 2019, 33(19): 3210-3218. doi: 10.11896/cldb.18080017
Li L T, Zang X B, Cao N. Recent progress of rechargeable zinc-manganese dioxide batteries[J]. Materials Review, 2019, 33(19): 3210-3218. doi: 10.11896/cldb.18080017
[10] 李俊豪, 冯斯桐, 张圣洁, 等. 高性能磷酸锰锂正极材料的研究进展[J]. 材料导报, 2019, 33(9): 2854-2861. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201917005.htm
Li J H, Feng S T, Zhang S J, et al. Research progress in high performance lithium manganese phosphate cathode materials[J]. Materials Review, 2019, 33(9): 2854-2861. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201917005.htm
[11] 李改云, 鲁安怀, 饶竹. 天然锰钾矿氧化降解水体中苯酚机理研究[J]. 岩石矿物学杂志, 2003, 22(4): 365-368. doi: 10.3969/j.issn.1000-6524.2003.04.010
Li G Y, Lu A H, Rao Z. Mechanism of oxidation and degradation of phenol in water by natural cryptomelane[J]. Acta Petrologica et Mineralogica, 2003, 22(4): 365-368. doi: 10.3969/j.issn.1000-6524.2003.04.010
[12] 杨欣, 鲁安怀, 李改云, 等. 天然锰钾矿处理印染废水实验研究[J]. 岩石矿物学杂志, 2003, 22(4): 370-373. https://www.cnki.com.cn/Article/CJFDTOTAL-YSKW200304010.htm
Yang X, Lu A H, Li G Y, et al. Oxidative decolorization of printing and dyeing wastewater by natural cryptomelane[J]. Acta Petrologica et Mineralogica, 2003, 22(4): 370-373. https://www.cnki.com.cn/Article/CJFDTOTAL-YSKW200304010.htm
[13] 殷珂, 陈瑞洋, 刘志明. 锰基氧化物上甲苯催化氧化的研究进展[J]. 材料导报, 2020, 34(12): 23051-23056. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB202023007.htm
Yin K, Chen R Y, Liu Z M. Catalytic removal of toluene over manganese-based oxide catalysts[J]. Materials Review, 2020, 34(12): 23051-23056. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB202023007.htm
[14] 黄慧娟, 尚莉莉, 马建锋, 等. 锰氧化物催化分解室内甲醛的研究进展[J]. 材料导报, 2019, 33(专辑34): 521-525. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2019S2105.htm
Huang H J, Shang L L, Ma J F, et al. Advances on catalytic oxidation of formaldehyde by manganese oxide[J]. Materials Review, 2019, 33(Special Issue 34): 521-525. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB2019S2105.htm
[15] 刘喆, 张晓岚, 蔡婷, 等. 用于甲醛催化氧化的锰基催化剂及协同效应的影响[J]. 化学进展, 2019, 31(2): 311-321. https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ2019Z1008.htm
Liu Z, Zhang X L, Cai T, et al. Catalytic oxidation of formaldehyde over manganese-based catalysts and the influence of synergistic effect[J]. Progress in Chemistry, 2019, 31(2): 311-321. https://www.cnki.com.cn/Article/CJFDTOTAL-HXJZ2019Z1008.htm
[16] Poyraz A S, Huang J P, Wu L J, et al. Potassium-based alpha-manganese dioxide nanofiber binderfree self-supporting electrodes: A design strategy for high energy density batteries[J]. Energy Technology, 2016, 4: 1-12. doi: 10.1002/ente.201500354
[17] 张成明, 庞鑫, 王永钊. 一维隐钾锰矿型二氧化锰的控制合成及其电化学性能[J]. 化学学报, 2018, 76(2): 133-137. https://www.cnki.com.cn/Article/CJFDTOTAL-HXXB201802008.htm
Zhang C M, Pang X, Wang Y Z. Controllable synthesis of one-dimensional cryptomelane-type manganese dioxide and its electrochemical performance[J]. Acta Chimica Sinica, 2018, 76(2): 133-137. https://www.cnki.com.cn/Article/CJFDTOTAL-HXXB201802008.htm
[18] Galezowski L, Recham N, Larcher D, et al. Microbially induced mineralization of layered Mn oxides electroactive in Li batteries[J]. Frontiers in Microbiology, 2020, 11, 2031. doi: 10.3389/fmicb.2020.02031
[19] Richmond W E, Fleischer M. Cryptomelane, a new name for the commonest of the "psilomelane" minerals[J]. American Mineralogist, 1942, 27(9): 607-610.
[20] Killick A M. The setting and style of manganese mineralization in the Constantiaberg Massif, Cape Peninsula, South Africa[J]. South African Journal of Geology, 2020: 123(4): 493-510. doi: 10.25131/sajg.123.0034
[21] Ismail S, Hushim S F S, Hussin H, et al. Chemical and mineralogical characterization of Malaysian low-grade manganese ore[J]. Periodico di Mineralogia, 2016, 85: 277-288.
[22] Peterson M J, Hapugoda S. Microhardness characterisation of manganese ore minerals—Implications for downstream processing[J]. Minerals Engineering, 2020, 157: 106537. doi: 10.1016/j.mineng.2020.106537
[23] 高翔, 鲁安怀, 秦善, 等. 天然锰钾矿晶体化学特征及其环境属性[J]. 岩石矿物学杂志, 2001, 20(4): 477-484. doi: 10.3969/j.issn.1000-6524.2001.04.022
Gao X, Lu A H, Qin S, et al. A study of crystal structural characteristics and environmental properties of natural cryptomelane[J]. Acta Petrologica et Mineralogica, 2001, 20(4): 477-484. doi: 10.3969/j.issn.1000-6524.2001.04.022
[24] 高翔, 鲁安怀, 郑辙, 等. 湖南湘潭锰矿锰钾矿——天然氧化物八面体分子筛(OMS-2)在HRTEM下的微结构特征研究[J]. 矿物岩石, 2005(3): 52-57. doi: 10.3969/j.issn.1001-6872.2005.03.011
Gao X, Lu A H, Zheng Z, et al. Natural oxide octahedral molecular sieve (OMS-2)—The study of the characters of microstructure by for HRTEM cryptomelane of Xiangtan manganese deposit, Hunan Province[J]. Journal of Mineralogy and Petrology, 2005(3): 52-57. doi: 10.3969/j.issn.1001-6872.2005.03.011
[25] Lu A H, Li Y. Reactivity of natural Mn oxide cryptomelane[M]. Washington D C: American Chemical Society, 2015: 89-106.
[26] 鲁安怀, 高翔, 秦善, 等. 锰钾矿(KxMn8-xO16): 天然活性八面体分子筛(OMS-2)[J]. 科学通报, 2003, 48(6): 97-100. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200306017.htm
Lu A H, Gao X, Qin S, et al. Cryptomelane (KxMn8-xO16): Natural active octahedral molecular sieve (OMS-2)[J]. Chinese Science Bulletin, 2003, 48(6): 97-100. https://www.cnki.com.cn/Article/CJFDTOTAL-KXTB200306017.htm
[27] 李增胜, 吴敏, 徐爽, 等. 应用电子探针技术研究山东金青顶金矿床碲化物特征[J]. 岩矿测试, 2018, 37(3): 266-274. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201709130150
Li Z S, Wu M, Xu S, et al. Application of electron microprobe to study the features of tellurides in the Jinqingding gold deposit, Shandong Province[J]. Rock and Mineral Analysis, 2018, 37(3): 266-274. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201709130150
[28] 王勇, 唐菊兴, 王立强. 西藏邦铺斑岩钼(铜)矿床钾硅酸盐化热液黑云母电子探针分析及早期成矿流体特征[J]. 岩矿测试, 2016, 35(4): 440-447. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.04.017
Wang Y, Tang J X, Wang L Q. EPMA Analysis of hydrothermal biotite from the Bangpu porphyry Mo-Cu deposit of Xizang, China and the characteristics of early ore-forming fluids[J]. Rock and Mineral Analysis, 2016, 35(4): 440-447. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.04.017
[29] 荆国强, 廉康, 胡菲菲, 等. 利用电子探针研究甘肃陇南赵家庄金矿载金矿物特征[J]. 岩矿测试, 2018, 37(5): 490-498. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201707170119
Jing G Q, Lian K, Hu F F, et al. Application of EPMA to study the characteristics of gold-bearing minerals in the Zhaojiazhuang gold deposit in Longnan, Gansu Province[J]. Rock and Mineral Analysis, 2018, 37(5): 490-498. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201707170119
[30] 张龙, 陈振宇, 田泽瑾, 等. 电子探针测年方法应用于粤北长江岩体的铀矿物年龄研究[J]. 岩矿测试, 2016, 35(1): 98-107. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.01.016
Zhang L, Chen Z Y, Tian Z J, et al. The application of electron microprobe dating method on uranium minerals in Changjiang granite, northern Guangdong[J]. Rock and Mineral Analysis, 2016, 35(1): 98-107. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.2016.01.016
[31] 李胜荣, 鲁安怀, 申俊峰, 等. 新世纪成因矿物学研究动向与进展: 纪念陈光远先生诞生100周年专辑[J]. 地学前缘, 2020, 27(5): 1-3. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202005002.htm
Li S R, Lu A H, Shen J F, et al. Trends and progress of genetic mineralogy in the new century: A special issue to commemorate the 100th anniversary of the birth of Chen Guangyuan[J]. Earth Science Frontiers, 2020, 27(5): 1-3. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY202005002.htm
[32] 赵东军, 鲁安怀, 王丽娟, 等. 广西下雷锰矿床中锰钾矿的矿物学特征[J]. 北京大学学报(自然科学版), 2005, 41(6): 859-868. doi: 10.3321/j.issn:0479-8023.2005.06.005
Zhao D J, Lu A H, Wang L J, et al. Mineralogical characteristics study of natural cryptomelane in the Xialei manganese deposit, Guangxi[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2005, 41(6): 859-868. doi: 10.3321/j.issn:0479-8023.2005.06.005
[33] Vafeas N A, Viljoen K S, Blignaut L C. Characterization of fibrous cryptomelane from the todorokite-cryptomelane mineral assemblage at the Sebilo mine, Northern Cape Province, South Africa[J]. The Canadian Mineralogist, 2018, 56(1): 65-76. doi: 10.3749/canmin.1700043
[34] 郝瑞霞, 关广岳. 下雷—湖润锰矿带氧化锰矿床的矿物组合及氧化机理[J]. 中国锰业, 1995, 13(1): 3-7. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMM501.000.htm
Hao R X, Guan G Y. The mineral assemblages and oxidizing mechanism of oxidized manganese deposits of Xialei—Hurun manganese mineralized zone[J]. China's Manganese Industry, 1995, 13(1): 3-7. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMM501.000.htm
[35] 颜代蓉, 李建威, 胡明安, 等. 广西下雷氧化锰矿床矿石特征及成因分析[J]. 地质科技情报, 2006, 25(3): 61-67. doi: 10.3969/j.issn.1000-7849.2006.03.011
Yan D R, Li J W, Hu M A, et al. Characteristics and genesis of supergene manganese ores in Xialei, Guangxi[J]. Geological Science and Technology Information, 2006, 25(3): 61-67. doi: 10.3969/j.issn.1000-7849.2006.03.011
[36] 赵立群, 周尚国, 伊海生, 等. 桂西南下雷锰矿床地球化学特征及沉积环境分析[J]. 地质与勘探, 2016, 52(1): 25-39. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKT201601003.htm
Zhao L Q, Zhou S G, Yi H S, et al. Geochemical characteristics and sedimentary environment of the Xialei manganese deposit in southwest Guangxi[J]. Geology and Exploration, 2016, 52(1): 25-39. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKT201601003.htm
[37] 朱建德, 朱恺军, 周尚国, 等. 下雷—东平锰矿带矿床特征和成因探讨[J]. 地质与勘探, 2016, 52(5): 846-853. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKT201605005.htm
Zhu J D, Zhu K J, Zhou S G, et al. Characteristics and genesis of deposits in the Xialei—Dongping manganese ore belt of Guangxi Province[J]. Geology and Exploration, 2016, 52(5): 846-853. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKT201605005.htm
[38] 金玺, 陈显锋. 广西大新县下雷锰矿地质特征与矿床成因探讨[J]. 矿产与地质, 2018, 32(3): 404-408. doi: 10.3969/j.issn.1001-5663.2018.03.004
J X, Chen X F. Geological characteristics and genesis of the Xialei Mn deposit in Daxin County, Guangxi[J]. Mineral Resources and Geology, 2018, 32(3): 404-408. doi: 10.3969/j.issn.1001-5663.2018.03.004
[39] 陆镜求, 莫俊晖. 下雷锰矿矿物相的分析及锰矿化学沉积模式研究[J]. 中国锰业, 2018, 36(1): 75-78. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMM201801022.htm
Lu J Q, Mo J H. Mineral phase analysis of manganese ore and chemical sedimentary model of manganese ore in Xialei Mn-mine[J]. China's Manganese Industry, 2018, 36(1): 75-78. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGMM201801022.htm
[40] Niu S, Zhao L, Lin X, et al. Mineralogical characterization of manganese oxide minerals of the Devonian Xialei manganese deposit[J]. Minerals, 2021, 11, 1243. https://doi.org/10.3390/min11111243. doi: 10.3390/min11111243
[41] Volokhin Y G, Mikhailik P E, Mikhailik E V. Minerals in manganese deposits of Belyaevsky Volcano, the Sea of Japan[J]. Russian Journal of Pacific Geology, 2020, 14(4): 340-362. doi: 10.1134/S1819714020040077
[42] Ling F T, Post J E, Heaney P J, et al. A multi-method characterization of natural terrestrial birnessites[J]. American Mineralogist, 2020, 105(6): 833-847. doi: 10.2138/am-2020-7303
[43] 李胜荣, 许虹, 申俊峰. 结晶学与矿物学[M]. 北京: 地质出版社, 2008: 1-346.
Li S R, Xu H, Shen J F. Crystallography and mineralogy[M]. Beijing: Geological Publishing House, 2008: 1-346.
[44] Khosravi M, Cathey H E, Mackinnon I. Comprehensive mineralogical study of Australian zeolites[J]. Microporous and Mesoporous Materials, 2020, 312(1-2): 110753.
[45] Tsai Y L, Huang E, Li Y H, et al. Raman spectroscopic characteristics of zeolite group minerals[J]. Minerals, 2021, 11(2): 167. doi: 10.3390/min11020167
[46] 李智, 王建英, 张向京, 等. 分子筛吸附法脱除VOCs的研究进展[J]. 煤炭与化工, 2019, 42(6): 121-125. https://www.cnki.com.cn/Article/CJFDTOTAL-HHGZ201906036.htm
Li Z, Wang J Y, Zhang X J, et al. Research progress of removing VOCs by molecular sieve adsorption method[J]. Coal and Chemical Industry, 2019, 42(6): 121-125. https://www.cnki.com.cn/Article/CJFDTOTAL-HHGZ201906036.htm
[47] Zhao L, Niu S, Li L, et al. Mineralogy and crystal structure studies of the manganese oxide in Xiangtan manganese deposit, South China and its preliminary application on absorption and catalysis of formaldehyde[C]//Goldschmidt Abstract. 2021. https://doi.org/10.7185/gold2021.5674.
-



 下载:
下载: