Influences of Nitrogen Cycle on Arsenic Enrichment in Shallow Groundwater from the Yellow River Alluvial Fan Plain
-
摘要:
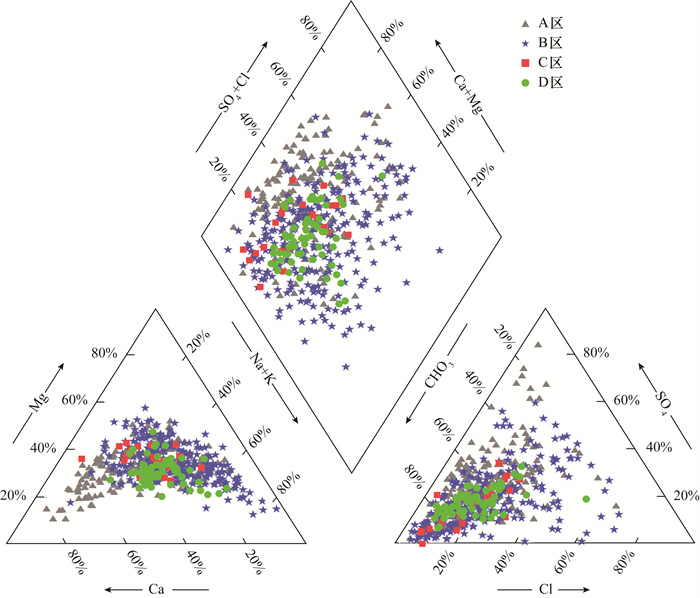
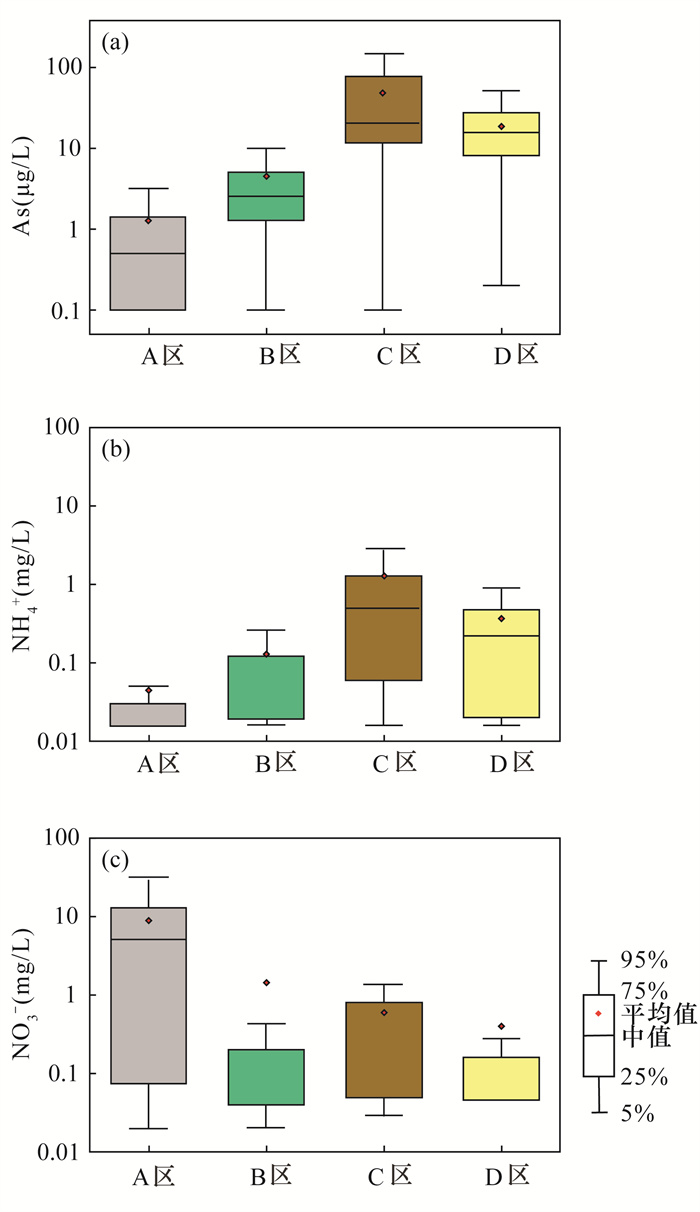
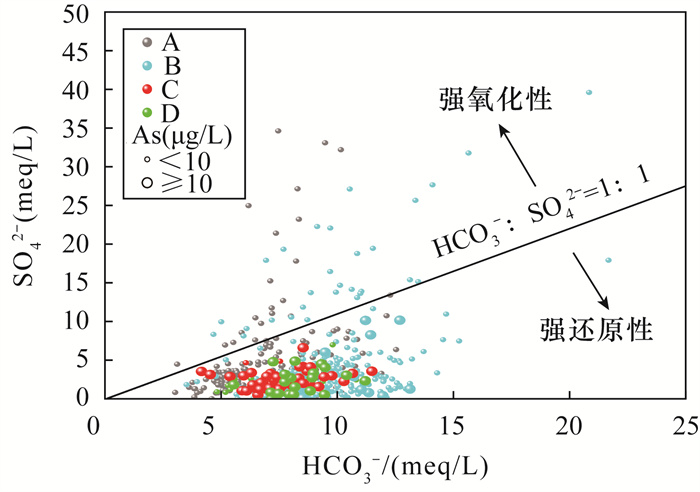
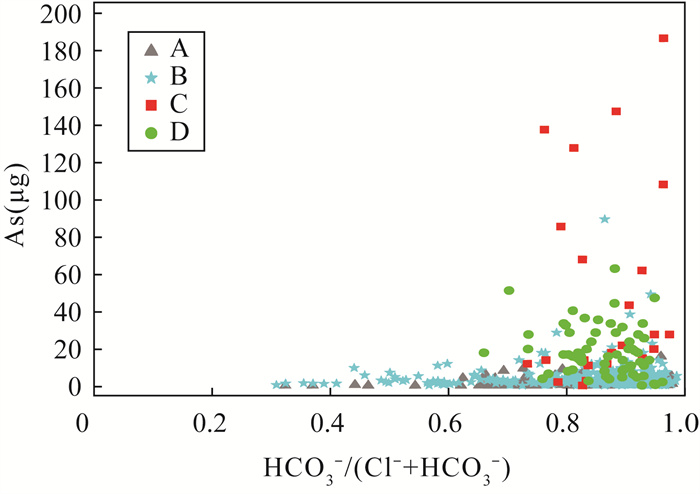
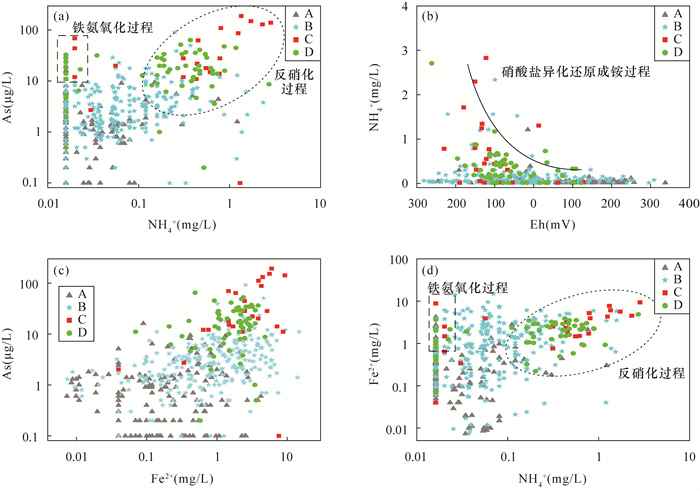
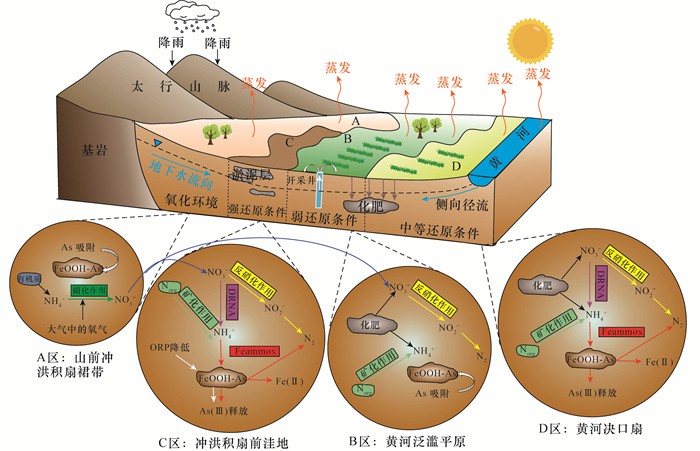
黄河冲积扇平原浅层地下水砷含量超标情况严重,豫北平原的主体是黄河冲洪积扇平原。全面了解豫北平原浅层地下水氮循环驱动下砷的富集模式,对地下水资源的可持续利用和居民健康至关重要。本文采集豫北平原513组浅层地下水样品,采用原子荧光光谱法测定砷含量,原子吸收光谱和离子色谱等方法进行全分析及微量元素分析,对该地区高砷地下水的水化学成分以及地下水中硝酸盐、氨氮与砷之间的相关关系进行探究,并研究了氮循环对地下水中砷迁移富集的影响。结果表明:研究区浅层地下水中砷浓度超标率为17.3%。不同沉积环境条件下氮的赋存形态和转化方式是砷富集的重要驱动因素。山前冲洪积扇裙带中经硝化作用产生大量NO3-,浓度平均值为9.3mg/L,为各区最高,同时砷浓度为各区最低,平均值为1.3μg/L,NO3-与砷浓度之间良好的负相关性表明硝化作用产生大量NO3-,不利于含砷氧化铁的溶解;NH4+含量较高的冲洪积扇前洼地及黄河决口扇地区,为高砷地下水的聚集地,两地地下水砷浓度平均值分别为49.7μg/L和18.9μg/L,超标率达到87.5%和71.4%。地下水中砷含量与NH4+之间良好的正相关关系表明,反硝化和硝酸异化还原成铵(DNRA)过程消耗了地下水中的NO3-,生成大量NH4+,促进吸附了砷的铁氧化物的还原溶解而导致砷释放到地下水中,形成了富集砷的环境。
Abstract:BACKGROUND Arsenic content in shallow groundwater of the Yellow River alluvial fan plain exceeds the standard. A comprehensive understanding of the arsenic enrichment mode driven by the nitrogen cycle of shallow groundwater in the northern Henan Plain is essential for the sustainable use of groundwater resources and the health of residents. The main area of the North Henan plain is the Yellow River alluvial fan plain. The sedimentary environment in the northern Henan plain is complex, and is influenced by the Yellow River breach, diversion and oscillation, as well as alluvial diluvial in the mountainous area around the basin. The distribution, migration and release mechanism of As, NH4+ and NO3- are quite different under different sedimentary environment conditions. The joint enrichment mechanism of the three is still unclear, which is worth further study.
OBJECTIVES To investigate the effect of nitrogen cycling on arsenic migration and enrichment in groundwater in the Northern Henan Plain.
METHODS 513 shallow groundwater samples were collected from the northern Henan Plain. Atomic fluorescence spectroscopy was used to determine the arsenic content, atomic absorption spectroscopy and ion chromatography, and other methods for major and trace element analysis. The correlation between nitrate, ammonia nitrogen and arsenic was investigated, and the influence of nitrogen cycle on the migration and enrichment of arsenic in groundwater was studied.
RESULTS The over-standard rate of arsenic concentration in shallow groundwater in the study area was 17.3%. The occurrence and transformation modes of nitrogen under different depositional environmental conditions were important driving factors for arsenic enrichment. A large amount of NO3- was produced by nitrification in the groundwater of the alluvial fan in the piedmont alluvial fan, which had the average concentration of 9.3mg/L and was the highest in the district. At the same time, the concentration of arsenic was the lowest in the district, with the average of 1.3μg/L. The good negative correlation between NO3- and As concentration indicated that nitrification produced a large amount of NO3-, which was not conducive to the dissolution of arsenic-containing iron oxide. The depressions in front of the alluvial fan and the Yellow River crater fan with higher NH4+ content were the gathering places of high-arsenic groundwater. The average concentration of arsenic in groundwater from these two areas was 49.7μg/L and 18.9μg/L, respectively, and the exceeding rate reached 87.5% and 71.4%. The good positive correlation between arsenic content and NH4+ in groundwater indicated that the process of denitrification and dissimilative reduction of nitric acid to ammonium (DNRA) consumed NO3- in groundwater and generated a large amount of NH4+, which promoted the reduction and dissolution of iron oxides that adsorbed arsenic, forming an environment rich in arsenic.
CONCLUSIONS The nitrogen cycle plays an important role in the migration and enrichment of arsenic. The migration and enrichment mode of arsenic driven by the nitrogen cycle provides a scientific basis for the treatment and supervision of groundwater with high arsenic content.
-

-
表 1 地下水主要化学组分统计特征
Table 1. Statistical characteristics of major chemical composition of groundwater
研究区(n=513) pH TDS (mg/L) Eh (mg/L) NO3- (mg/L) NH4+ (mg/L) Fe2+ (mg/L) As (μg/L) 最小值 6.1 271.3 -281.0 <0.01 <0.016 <0.04 <0.1 最大值 8.4 7825.0 337.0 239.4 2.82 14.8 190.0 平均值 7.4 1012.2 -9.0 3.8 0.18 1.4 7.4 中值 7.4 797.0 -23.0 0.1 0.03 0.7 2.2 SD 0.3 782.0 122.9 13.4 0.69 1.8 17.5 CV 0.04 0.8 -13.7 3.6 3.8 1.3 2.4 A区(n=164) pH TDS (mg/L) Eh (mg/L) NO3- (mg/L) NH4+ (mg/L) Fe2+ (mg/L) As (μg/L) 最小值 6.9 271.3 -245.0 <0.01 <0.016 <0.04 <0.1 最大值 8.3 5561.0 337.0 68.2 1.2 4.2 16.0 平均值 7.4 1006.0 83.4 9.3 0.04 0.4 1.3 中值 7.4 800.5 96.0 5.2 0.02 0.1 0.5 SD 0.3 699.2 101.9 12.9 0.12 0.7 2.1 CV 0.04 0.7 1.2 1.4 2.6 1.7 1.7 B区(n=262) pH TDS (mg/L) Eh (mg/L) NO3- (mg/L) NH4+ (mg/L) Fe2+ (mg/L) As (μg/L) 最小值 6.1 326.0 -281.0 <0.01 <0.016 <0.04 <0.1 最大值 8.4 7825.0 308.0 239.4 2.3 14.8 91.0 平均值 7.4 1100.4 -44.5 1.4 0.13 1.6 4.6 中值 7.4 844.5 -53.5 0.1 0.05 0.9 2.6 SD 0.3 918.2 113.6 14.8 0.26 2.0 7.6 CV 0.05 0.8 -2.6 10.3 2.1 1.2 1.7 C区(n=24) pH TDS (mg/L) Eh (mg/L) NO3- (mg/L) NH4+ (mg/L) Fe2+ (mg/L) As (μg/L) 最小值 7.0 431.0 -229.0 0.03 <0.016 <0.04 <0.1 最大值 8.0 1151.5 127.0 4.7 2.82 9.3 190.0 平均值 7.5 752.9 -105.7 0.6 1.27 3.6 49.7 中值 7.4 738.0 -124.0 0.3 0.50 2.8 21.0 SD 0.2 221.8 78.9 1.0 2.81 2.7 55.2 CV 0.03 0.3 -0.7 1.6 2.2 0.7 1.1 D区(n=63) pH TDS (mg/L) Eh (mg/L) NO3- (mg/L) NH4+ (mg/L) Fe2+ (mg/L) As (μg/L) 最小值 6.9 361.0 -261.0 0.05 <0.016 <0.04 <0.1 最大值 7.9 1990.4 121.0 17.6 2.7 4.8 64.0 平均值 7.5 760.1 -64.9 0.4 0.34 1.9 18.9 中值 7.6 690.8 -85.0 0.04 0.22 2.0 16.0 SD 0.2 271.7 78.2 2.2 0.40 1.0 13.6 CV 0.03 0.4 -1.2 5.5 1.2 0.6 0.7 注:SD为标准差; CV为变异系数; CV=SD/平均值。 -
[1] Daniele P, Stefano G, Eleonora F, et al. Arsenic-fluoride co-contamination in groundwater: Background and anomalies in a volcanic-sedimentary aquifer in central Italy[J]. Journal of Geochemical Exploration, 2020, 217. http://www.sciencedirect.com/science/article/pii/S0375674220301837
[2] Podgorski J, Berg M. Global threat of arsenic in groundwater[J]. Science, 2020, 368(6493): 845-850. doi: 10.1126/science.aba1510
[3] 杨文蕾, 沈亚婷. 水稻对砷吸收的机理及控制砷吸收的农艺途径研究进展[J]. 岩矿测试, 2020, 39(4): 475-492. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.202004160052
Yang W L, Shen Y T. A review of research progress on the absorption mechanism of arsenic and agronomic pathwaysto control arsenic absorption[J]. Rock and Mineral Analysis, 2020, 39(4): 475-492. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.202004160052
[4] 郭华明, 倪萍, 贾永锋, 等. 原生高砷地下水的类型、化学特征及成因[J]. 地学前缘, 2014, 21(4): 1-12. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201404002.htm
Guo H M, Ni P, Jia Y F, et al. Chemical characteristics and genesis of geogenic high-arsenic groundwater in the world[J]. Earth Science Frontiers, 2014, 21(4): 1-12. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201404002.htm
[5] Cao W G, Guo H M, Zhang Y L, et al. Controls of paleo-channels on groundwater arsenic distribution in shallow aquifers of alluvial plain in the Hetao Basin, China[J]. Science of the Total Environment, 2018, 613-614(1): 958-968. http://www.sciencedirect.com/science/article/pii/s0048969717325330
[6] Kumar M, Das A, Das N, et al. Co-occurrence perspective of arsenic and fluoride in the groundwater of Diphu, Assam, northeastern India[J]. Chemosphere, 2016, 150: 227-238. doi: 10.1016/j.chemosphere.2016.02.019
[7] Pi K F, Wang Y X, Xie X J, et al. Hydrogeochemistry of co-occurring geogenic arsenic, fluoride and iodine in groundwater at Datong Basin, northern China[J]. Journal of Hazardous Materials, 2015, 300: 652-661. doi: 10.1016/j.jhazmat.2015.07.080
[8] Wen D G, Zhang F C, Zhang E Y, et al. Arsenic, fluoride and iodine in groundwater of China[J]. Journal of Geochemical Exploration, 2013, 135: 1-21. doi: 10.1016/j.gexplo.2013.10.012
[9] Janardhana R N. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies[J]. Environmental Research, 2021, 203: 111782. http://www.sciencedirect.com/science/article/pii/S0013935121010768
[10] 郭华明, 郭琦, 贾永锋, 等. 中国不同区域高砷地下水化学特征及形成过程[J]. 地球科学与环境学报, 2013, 35(3): 83-96. https://www.cnki.com.cn/Article/CJFDTOTAL-XAGX201303010.htm
Guo H M, Guo Q, Jia Y F, et al. Chemical characteristics and geochemical processes of high arsenic groundwater in different regions of China[J]. Journal of Earth Sciences and Environment, 2013, 35(3): 83-96. https://www.cnki.com.cn/Article/CJFDTOTAL-XAGX201303010.htm
[11] 董会军, 董建芳, 王昕洲, 等. pH值对HPLC-ICP-MS测定水体中不同形态砷化合物的影响[J]. 岩矿测试, 2019, 38(5): 510-517. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201808230096
Dong H J, Dong J F, Wang X Z, et al. Effect of pH on determination of various arsenic species in water by HPLC-ICP-MS[J]. Rock and Mineral Analysis, 2019, 38(5): 510-517. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201808230096
[12] Sahoo P K, Zhu W, Kim S H, et al. Relations of arsenic concentrations among groundwater, soil and paddy from an alluvial plain of Korea[J]. Geosciences Journal, 2013, 17(3): 363-370. doi: 10.1007/s12303-013-0031-1
[13] 吴昆明, 郭华明, 魏朝俊. 改性磁铁矿对水体中砷的吸附特性研究[J]. 岩矿测试, 2017, 36(6): 624-632. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201709110147
Wu K M, Guo H M, Wei C J. Adsorption characteristics of arsenic in water by modified magnetite[J]. Rock and Mineral Analysis, 2017, 36(6): 624-632. http://www.ykcs.ac.cn/article/doi/10.15898/j.cnki.11-2131/td.201709110147
[14] Stüben D, Berner Z, Chandrasekharam D, et al. Arsenic enrichment in groundwater of West Bengal, India: Geochemical evidence for mobilization of As under reducing conditions[J]. Applied Geochemistry, 2003, 18(9): 1417-1434. doi: 10.1016/S0883-2927(03)00060-X
[15] Ravenscroft P, Burgess W G, Ahmed K M, et al. Arsenic in groundwater of the Bengal Basin, Bangladesh: Distribution, field relations, and hydrogeological setting[J]. Hydrogeology Journal, 2006, 13(5-6): 727-751.
[16] Kurosawa K, Egashira K, Tani M, et al. Variation in arsenic concentration relative to ammonium nitrogen and oxidation reduction potential in surface and groundwater[J]. Communications in Soil Science and Plant Analysis, 2008, 39(9-10): 1467-1475. doi: 10.1080/00103620802004318
[17] Canfield D E, Glazer A N, Falkowski P G. The evolution and future of Earth's nitrogen cycle[J]. Science, 2010, 330(6001): 192-196. doi: 10.1126/science.1186120
[18] Berner R A. Geological nitrogen cycle and atmospheric N2 over phanerozoic time[J]. Geology, 2006, 34(5): 413-415. doi: 10.1130/G22470.1
[19] Norrman J, Sparrenbom C J, Berg M, et al. Tracing sources of ammonium in reducing groundwater in a well field in Hanoi (Vietnam) by means of stable nitrogen isotope (δ15N) values[J]. Applied Geochemistry, 2015, 61(15): 248-258.
[20] 祝贤彬. 砷氧化还原微生物催化的硝酸盐转化及其对环境的影响[D]. 北京: 中国地质大学(北京), 2020.
Zhu X B. Nitrate transformations catalyzed by the arsenic redox microorganisms and their environmental influences[D]. Beijing: China University of Geosciences(Beijing), 2020.
[21] 贾正雷. 土壤砷和氮含量的空间变异及其相互关系研究[D]. 广州: 华南农业大学, 2016.
Jia Z L. Study on spatial variability and relationship of soil arsenic and soil nitrogen[D]. Guangzhou: South China Agricultural University, 2016.
[22] Gao Z P, Weng H C, Guo H M. Unraveling influences of nitrogen cycling on arsenic enrichment in groundwater from the Hetao Basin using geochemical and multi-isotopic approaches[J]. Journal of Hydrology, 2021, 595: 125981. doi: 10.1016/j.jhydrol.2021.125981
[23] Smith R L, Kent D B, Repert D A, et al. Anoxic nitrate reduction coupled with iron oxidation and attenuation of dissolved arsenic and phosphate in a sand and gravel aquifer[J]. Geochimica et Cosmochimica Acta, 2017, 196(1): 102-120.
[24] Karunanidhi D, Aravinthasamy P, Subramani T, et al. Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River Basin, South India[J]. Human & Ecological Risk Assessment, 2019, 25(1-2): 250-270. http://www.onacademic.com/detail/journal_1000041611805999_e41a.html
[25] Singh G, Rishi M S, Herojeet R, et al. Evaluation of groundwater quality and human health risks from fluoride and nitrate in semi-arid region of northern India[J]. Environmental Geochemistry and Health, 2020, 42(7): 1833-1862. doi: 10.1007/s10653-019-00449-6
[26] Böhlke J K, Smith R L, Miller D N. Ammonium transport and reaction in contaminated groundwater: Application of isotope tracers and isotope fractionation studies[J]. Water Resources Research, 2007, 42(5): W05411(1-19).
[27] Utom A U, Werban U, Leven C, et al. Groundwater nitrification and denitrification are not always strictly aerobic and anaerobic processes, respectively: An assessment of dual-nitrate isotopic and chemical evidence in a stratified alluvial aquifer[J]. Biogeochemistry, 2020, 147(2): 211-223. doi: 10.1007/s10533-020-00637-y
[28] Savard M M, Paradis D, Somers G, et al. Winter nitrification contributes to excess NO3- in groundwater of an agricultural region: A dual-isotope study[J]. Water Resources Research, 2008, 43(6): W06422(1-10). http://dx.doi.org/10.1029/2006wr005469
[29] Rivett M O, Buss S R, Morgan P, et al. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes[J]. Water Research, 2008, 42(16): 4215-4232. doi: 10.1016/j.watres.2008.07.020
[30] Singha S, Anilb A G, Kumarc V, et al. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation[J]. Chemosphere, 2021, 287(1): 131996.
[31] Rütting T, Huygens D, Müller C, et al. Functional role of DNRA and nitrite reduction in a pristine south Chilean Nothofagus forest[J]. Biogeochemistry, 2008, 90(3): 243-258. doi: 10.1007/s10533-008-9250-3
[32] 杨杉, 吴胜军, 蔡延江, 等. 硝态氮异化还原机制及其主导因素研究进展[J]. 生态学报, 2016, 36(5): 1224-1232. https://www.cnki.com.cn/Article/CJFDTOTAL-STXB201605005.htm
Yang S, Wu S J, Cai Y J, et al. The synergetic and competitive mechanism andthe dominant factors of dissimilatory nitrate reduction processes: A review[J]. Acta Ecologica Sinica, 2016, 36(5): 1224-1232. https://www.cnki.com.cn/Article/CJFDTOTAL-STXB201605005.htm
[33] Yang W H, Weber K A, Silver W L. Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction[J]. Nature Geoscience, 2012, 5(8): 538-541. doi: 10.1038/ngeo1530
[34] Ding B J, Chen Z H, Li Z K, et al. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from ecosystem habitats in the Taihu estuary region[J]. Science of the Total Environment, 2019, 662(1): 600-606. http://www.ncbi.nlm.nih.gov/pubmed/30699380
[35] Ding L J, An X L, Li S, et al. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence[J]. Environmental Science & Technology, 2014, 48(18): 10641-10647. http://www.onacademic.com/detail/journal_1000036708963710_7935.html
[36] Chen Y, Syvitski J P, Gao S, et al. Socio-economic impacts on flooding: A 4000-year history of the Yellow River, China[J]. Ambio, 2012, 41(7): 682-698. doi: 10.1007/s13280-012-0290-5
[37] Guo H M, Liu C, Lu H, et al. Pathways of coupled arsenic and iron cycling in high arsenic groundwater of the Hetao Basin, Inner Mongolia, China: An iron isotope approach[J]. Geochimica et Cosmochimica Acta, 2013, 112(1): 130-145. http://www.researchgate.net/profile/Huaming_Guo/publication/236273971_Pathways_of_coupled_arsenic_and_iron_cycling_in_high_arsenic_groundwater_of_the_Hetao_basin_Inner_Mongolia_China_An_iron_isotope_approach/links/5b8b40cb299bf1d5a737f587/Pathways-of-coupled-arsenic-and-iron-cycling-in-high-arsenic-groundwater-of-the-Hetao-basin-Inner-Mongolia-China-An-iron-isotope-approach.pdf
[38] Guo H M, Zhang Y, Jia Y F, et al. Dynamic behaviors of water levels and arsenic concentration in shallow groundwater from the Hetao Basin, Inner Mongolia[J]. Journal of Geochemical Exploration, 2013, 135: 130-140. doi: 10.1016/j.gexplo.2012.06.010
[39] Stachowicz M, Hiemstra T, Riemsdijk W H. Arsenic-bicarbonate interaction on goethite particles[J]. Environmental Science & Technology, 2007, 41(16): 5620-5625. http://pubs.acs.org/doi/suppl/10.1021/es063087i/suppl_file/es063087isi20070419_090234.pdf
[40] DeVore C L, Rodriguez-Freire L, Mehdi-Ali A, et al. Effect of bicarbonate and phosphate on arsenic release from mining-impacted sediments in the Cheyenne River watershed, South Dakota, USA[J]. Environmental Science Processes & Impacts, 2019, 21(3): 456-468.
[41] Gao X B, Su C L, Wang Y X, et al. Mobility of arsenic in aquifer sediments at Datong Basin, northern China: Effect of bicarbonate and phosphate[J]. Journal of Geochemical Exploration, 2013, 135: 93-103. doi: 10.1016/j.gexplo.2012.09.001
[42] Anawar H M, Akai J, Sakugawa H. Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater[J]. Chemosphere, 2004, 54(6): 753-762. doi: 10.1016/j.chemosphere.2003.08.030
[43] Appelo C A J, Van Der Weiden M J J, Tournassat C, et al. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic[J]. Environmental Science & Technology, 2002, 36(14): 3096-3103.
[44] Smedley P L, Kinniburgh D G. A review of the source, behaviour and distribution of arsenic in natural waters[J]. Applied Geochemistry, 2002, 17(5): 517-568.
-




 下载:
下载: