Characteristics of Lignin-derived Phenolic Compounds in Arid Lake, Northeastern China and Climatic Implications
-
摘要:
木质素广泛分布于维管植物,经分解生成的酚类化合物可示踪有机质来源、评估木质素降解程度,进而用于反演古环境与古气候变化。采用合适的分析方法有效地分解木质素是推断母源植物类型、降解程度的技术基础,常规方法是木质素经碱(或酸)解后,利用气相色谱-质谱法(GC-MS)分析酚类单体化合物,但分解、提取过程复杂、易引入杂质。热裂解技术可在高温下快速分解有机质,裂解产物可通过GC-MS进行在线分析,具有用样量少、有机质提取比例高、重现性好、操作便捷的特点。本文选择地处亚洲夏季风影响区域的边缘的内蒙伊和沙日乌苏湖,采用热裂解GC-MS(Py-GC/MS)技术,对湖泊沉积物进行裂解分析,在对裂解温度(450℃、550℃和650℃)进行了优化的基础上,识别了21种酚类化合物,包括:4-甲基苯酚、2-乙基苯酚等9种烷基酚类(PHs),4-乙基-2-甲氧基苯酚、4-乙烯基-2, 6-二甲氧基苯酚等9种烷基酚类(PHs)和12种甲氧基酚类(LGs)。结合沉积岩心样品AMS 14C年龄的分析结果,6.7ka以来沉积物中酚类化合物总量、PHs和LGs的变化趋势总体一致,呈现出6.7~4.0ka相对含量较高、4.0ka以来相对含量较低的特征。不同于PHs中邻(o-)-PHs、间(m-)-PHs、对(p-)-PHs的变化趋势与总量一致;但不同取代特征的LGs相对含量变化趋势存在差异,p-LGs在5.4ka前后就出现含量显著下降,3.8ka以来维持较低水平。根据微生物对木质素的“去甲基/去甲氧基”氧化反应途径,对位取代酚类化合物比值(p-PHs/p-LGs)可作为陆生高等植物降解指标,该值越大微生物降解作用越强。将p-PHs/p-LGs指标应用于伊和沙日乌苏沉积物样品结果显示,6.7ka以来p-PHs/p-LGs与正构烷烃单体碳同位素δ13C27~33变化趋势一致(R=-0.77),间接地指示了有效降水变化。即6.7ka以来气候整体转湿,区内陆生高等植物占据优势,充足的水分和有机质为微生物提供了适宜的生存环境和相对稳定的营养来源,降解作用整体呈增强趋势;6.3~5.5ka和4.1~3.6ka期间有效湿度降低,微生物对木质素的降解作用相对减弱。p-PHs/p-LGs指标对应了呼伦贝尔地区湿度变化特征,揭示了干旱-半干旱地区微生物降解与有效湿度变化的相关性,为探讨陆地生态系统对东亚季风北部边缘区气候变化的响应提供科学依据。
-
关键词:
- 热裂解-气相色谱-质谱法 /
- 伊和沙日乌苏湖 /
- 木质素酚类单体化合物 /
- 微生物降解 /
- 古气候
Abstract:BACKGROUND Lignin is widely distributed in vascular plants, and lignin-derived phenolic compounds generated by decomposition could provide information on the source of organic matter and the degradation degree of lignin. The conventional method for lignin deconstruction is complex and involves lignin hydrolysis via alkaline/acid chemical reagents. The analytical technique pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS) breaks the chemical bonds of large molecule compounds by instantaneous high temperature to generate a series of small molecule compounds without introducing pretreatment methods such as chemical extractions, realizing the online analysis of complex organic matter that is not easy to be gasified. This technique is characterized by low sample volume, high organic matter extraction ratio, good reproducibility, and convenient operation. It has been shown that the high-temperature cracking products of peat and lake sediments are similar to the results of traditional CuO oxidative decomposition. The distribution characteristics of phenolic compounds indicate the vegetation type and organic matter degradation characteristics. However, the optimization of analytical methods, application of environmental indication significance, and comparative studies of different matrix samples are still needed.
OBJECTIVES (1) Investigate suitable analytical methods for decomposing lignin in lake sediment samples and identify pyrolytic phenolic compounds in the sediments of Yiheshariwusu Lake in the northeast semi-arid region of China (Fig.E.1A, B). (2)Discuss the distribution characteristics of phenolic compounds in the sediments of Yiheshariwusu Lake. (3) Reveal the correlation between pyrolytic lignin phenols and regional climate change in the study area by combining traditional climate proxies, and provide an effective indicator for interpreting the response of terrestrial ecosystems to global climate change.
METHODS (1) Analytical method: An optimized analytical method of Py-GC/MS was established and applied to evaluate lignin-derived phenolic compounds in typical arid lake sediment. Samples were heated to 650℃ for 20s (heating rate 20℃/ms) and pyrolysis products were injected into the gas chromatography (GC) system in split mode, then separated in a nonpolar, low-bleed fused silica column (DB-1MS, 60m, 0.25mm i.d., 0.25μm film thickness, J&W). The GC oven program was set to increase from 40 to 320℃ at a rate of 4℃/min, and left at 320℃ for 18min. With internal electron ionization and ion trapping, the compounds were fragmented and identified in full scan mode (40-450amu). Blank and duplicate samples were analysed for quality control.
(2) Establishment of climatic proxy: Yiheshariwusu was selected as a typical arid lake and pyrolytical phenolic compounds of sediment cores were analysed. Historical variation of phenolic compound combing with radiocarbon dating results were revealed. According to "demethyl/demethoxy" oxidation reaction pathway of microorganisms to lignin, indicator related to degradation degree of lignin was established, and by comparing the indicators with conventional climate proxies previously published in the region, correlations between the indicators and climate features such as effective precipitation can be explored.
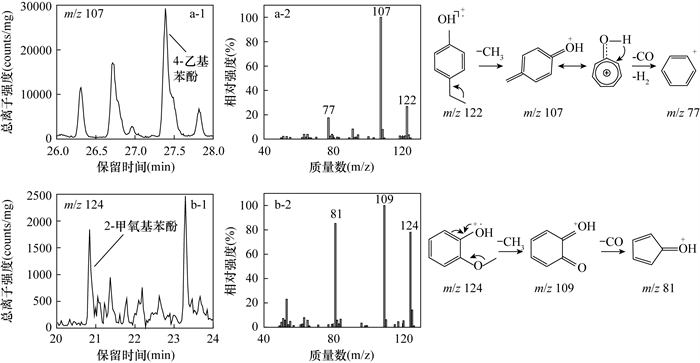
RESULTS (1) Py-GC/MS analysis method for phenolic compounds was optimized. Phenolic compounds in the total pyrolytic products of sediments were categorized into 2 groups according to the type of functional group: alkyl-phenols (phenol compounds, PHs) and methoxy-phenols (lignin monomer compounds, LGs), which are further divided into o-, m- and p-compounds according to the position of the substituent on the benzene ring structure. Based on fine characterization of organic matter composition in the sediments of Yiheshariwusu Lake in Inner Mongolia, 21 phenolic compounds were identified and analyzed, including 9 PHs and 12 LGs (Table 1). Pyrolysis temperature is the main factor affecting the results of Py-GC/MS analysis of sediment organic matter fingerprinting. By discussing the effect of different pyrolysis temperatures on the distribution characteristics of the total pyrolytic products at 450℃, 550℃ and 650℃, it was determined that the relative concentration of lignin phenolic compounds increased significantly with increasing pyrolysis temperature. The ether bond (C—O—C) connecting the lignin skeleton structure benzene propane structural unit was further broken as the temperature was increased from 450℃ to 650℃. The relative concentration of phenolic compounds in the pyrolysis compounds reached the highest proportion (16.46%), while the proportion of aromatic hydrocarbons and aliphatic hydrocarbons increased by 3.98% and 10.26%, respectively. The natural macromolecules, which are not easily vaporized, are gradually cleaved into smaller ionic fragments of phenolic compounds under high pyrolysis temperature. As the cleavage temperature increases to 650℃, the flux of phenolic compounds into the chromatographic system increases and the gas chromatographic response is gradually enhanced, with the phenolic compounds reaching the highest ionic intensity response. At the same time, the unit peak area, shape and signal-to-noise ratio were all improved, which improved the accuracy of phenolic compound identification and analysis.
(2) Distribution characteristics of phenolic compounds in Yiheshariwusu Lake were discussed. According to AMS 14C age data, historical variation of total phenolic compounds, PHs and LGs in lake sediment are generally consistent since 6.7ka, showing the characteristics of high relative concentration of 6.7-4.0ka and low concentration since 4.0ka. The variation characteristics of o-PHs, m-PHs, and p-PHs are consistent with total PHs, yet the change characteristics of p-LGs and LGs are different, the relative concentration of p-LGs decreased significantly near 5.4ka and remained at a low level since 3.8ka (average relative concentration of 0.29%). Combined with the lithological characteristics of sediment cores, relative concentration of total phenolic compounds and PHs decreased significantly around 4.0ka, probably due to the change of sedimentary lithology from sand to clay with smaller grain sizes during 4.0-3.8ka, where compounds with smaller molecular weights were preserved in the sediments, and the relative concentration of aliphatic hydrocarbons (e.g., n-alkanes, n-alkenes) and aromatic hydrocarbons significantly increased.
(3) Environmental indication significance of phenolic compounds was studied. According to previous studies of free n-alkanes distribution proxies (e.g., ACL23-33), higher relative concentration in lake sediments of p-PHs indicated major herbaceous source of lignin, however, significant differences in the mean relative concentration of p-PHs and LGs indicated significant microbial degradation of organic matter. The mean value of p-PHs/p-LGs for Yiheshariwusu Lake sediments was 16.41 (n=31, range of 4.36-37.31), showing an overall increasing trend since 6.7ka, reflecting a gradual increase in microbial activities. p-PHs/p-LGs showed a consistent trend and negative correlation with δ13C27-33 (n=31, R=-0.77, p < 0.01), meanwhile, variation of p-PHs/p-LGs positively correlated with the trend of increasing pollen of Chenopodiaceae and Poaceae in Hulun Lake sediment (n=31, R=0.54, p < 0.01), and on a larger scale, p-PHs/p-LGs are consistent and positively correlated with the gradual increase in the standardized precipitation index since 6.7ka in the northern hemisphere mid-latitudes (n=31, R=0.62, p < 0.01) (Fig.E.1C).
CONCLUSIONS The suitable pyrolysis temperature for Py-GC/MS analysis of phenolic compounds in the sediments of Yiheshariwusu Lake is 650℃. The value of degradation index p-PHs/p-LGs corresponds to the degree of lignin degradation, and the larger the value, the stronger the microbial degradation. Applying the p-PHs/p-LGs index to the sediment samples of Yiheshariwusu Lake, the result show that degradation index (p-PHs/p-LGs) and the carbon isotope of free n-alkanes δ13C27-33 has solid correlation since 6.7ka, indirectly indicating the change of effective precipitation, as the climate turned wet generally since 6.7ka, with terrestrial higher plants dominant, humid climate and sufficient organic matter provided a suitable living environment and relatively stable nutrient source for microorganisms, and the degradation generally increased. Since effective precipitation decreased during 6.3-5.5ka and 4.1-3.6ka, the degradation of lignin by microorganisms was relatively weakened. The p-PHs/p-LGs index corresponds to the characteristics of effective precipitation in the Hulun Buir region, revealing the correlation between microbial degradation and humidity change in arid and semi-arid regions. These findings provide a scientific basis for exploring the response of terrestrial ecosystems to climate change in the northern marginal region of the East Asian monsoon.
-

-
表 1 伊和沙日乌苏湖沉积物裂解产物中酚类化合物
Table 1. Pyrolytic phenolic compounds in sediment of Yiheshariwusu Lake
代号 化合物名称 保留时间(min) 化学式 分子量 特征离子(m/z) PH1 苯酚
Phenol20.14 C6H6O 94 94 PH2 2-甲基苯酚
2-Methylphenol23.02 C7H8O 108 107, 108 PH3 苯乙酮
Acetophenone23.22 C8H8O 120 105, 77 PH4 4-甲基苯
4-Methylphenol23.87 C7H8O 108 107, 108 PH5 2-乙基苯酚
2-Ethylphenol26.26 C8H10O 122 107, 122 PH6 3-乙基苯酚
3-Ethylphenol26.67 C8H10O 122 107, 122 PH7 4-乙基苯酚
4-Ethylphenol27.36 C8H10O 122 107, 122 PH8 2-乙基-6-甲基苯酚
2-Ethyl-6-methylphenol29.59 C9H12O 136 121, 136 PH9 2-乙基-5-甲基苯酚
2-Ethyl-5-methylphenol29.97 C9H12O 136 121, 136 LG1 2-甲氧基苯酚
2-Methoxyphenol (Guaiacol)23.22 C7H8O2 124 109, 124 LG2 4-甲氧基苯酚
Methoxyphenol24.24 C7H8O2 124 109, 124 LG3 5-甲基-2-甲氧基苯酚
Methoxy-5-methylphenol
(5-Methylguaiacol)28.07 C8H10O2 138 123, 138 LG4 4-乙基-2-甲氧基苯酚
4-Ethyl-2-methoxyphenol
(4-Ethylguaiacol)31.42 C9H12O2 152 137, 152 LG5 4-乙烯基-2-甲氧基苯酚
4-Vinyl-2-methoxyphenol
(4-Vinylguaiacol)32.54 C9H10O2 150 135, 150 LG6 2, 6-二甲氧基苯酚
2, 6-dimethoxyphenol (Syringol)33.46 C8H10O3 154 154, 139 LG7 4-(2-丙烯基)-2-甲氧基苯酚
4-(2-Propenyl)-2-methoxyphenol (Eugenol)37.06 C10H12O2 164 164, 149 LG8 4-乙酰基-2-甲氧基苯酚
4-Acetyl-2-methoxyphenol (4-Acetylguaiacol)37.85 C9H10O3 166 151, 166 LG9 4-乙基-2, 6-二甲氧基苯酚
4-Ethyl-2, 6-dimethoxyphenol (4-Ethylsyringol)39.19 C10H14O3 182 167, 182 LG10 4-乙烯基-2, 6-二甲氧基苯酚
4-Vinyl-2, 6-dimethoxyphenol (4-Vinylsyringol)40.28 C10H12O3 180 165, 180 LG11 4-羟基-3, 5-二甲氧基苯甲醛
4-Hydroxy-3, 5-dimethoxybenzaldehyde (syringaldehyde)43.06 C9H10O4 182 182, 181 LG12 4-(1-丙烯基)-2, 6-二甲氧基苯酚
4-(1-Propenyl)-2, 6-dimethoxyphenol
(4-Propenylsyringol)44.21 C11H14O3 194 194, 91 -
[1] Vanholme R, Demedts B, Morreel K, et al. Lignin biosyn-thesis and structure[J]. Plant Physiology, 2010, 153(3): 895-905. doi: 10.1104/pp.110.155119
[2] Mitra S, Bianchi T S, Guo L, et al. Terrestrially derived dissolved organic matter in the chesapeake bay and the Middle Atlantic Bight[J]. Geochimica et Cosmochimica Acta, 2000, 64(20): 3547-3557. doi: 10.1016/S0016-7037(00)00450-6
[3] Jex C N, Pate G H, Blyth A J, et al. Lignin biogeochemistry: From modern processes to Quaternary archives[J]. Quaternary Science Reviews, 2014, 87: 46-59. doi: 10.1016/j.quascirev.2013.12.028
[4] Ward N D, Krusche A V, Sawakuchi H O, et al. The compositional evolution of dissolved and particulate organic matter along the lower Amazon River-Óbidos to the ocean[J]. Marine Chemistry, 2015, 177: 244-256. doi: 10.1016/j.marchem.2015.06.013
[5] 冯晓娟, 王依云, 刘婷, 等. 生物标志物及其在生态系统研究中的应用[J]. 植物生态学报, 2020, 44(4): 384-394. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWSB202004010.htm
Feng X J, Wang Y Y, Liu T, et al. Biomarkers and their applications in ecosystem research[J]. Chinese Journal of Plant Ecology, 2020, 44(4): 384-394. https://www.cnki.com.cn/Article/CJFDTOTAL-ZWSB202004010.htm
[6] 凌媛, 王永, 王淑贤, 等. 生物标志物在海洋和湖泊古生态系统和生产力重建中的应用[J]. 地学前缘, 2022, 29(2): 327-342. doi: 10.13745/j.esf.sf.2021.10.35
Ling Y, Wang Y, Wang S X, et al. Application of biomarkers in reconstructing marine and lacustrine paleoecosystems and paleoproductivity: A review[J]. Earth Science Frontiers, 2022, 29(2): 327-342. doi: 10.13745/j.esf.sf.2021.10.35
[7] Goñi M A, Montgomery S. Alkaline CuO oxidation with a microwave digestion system: Lignin analyses of geochemical samples[J]. Analytical Chemistry, 2000, 72(14): 3116-3121. doi: 10.1021/ac991316w
[8] Klein K, Gross-Schm lders M, Alewell C, et al. Chapter three-heating up a cold case: Applications of analytical pyrolysis GC/MS to assess molecular biomarkers in peat[M]//Advances in Agronomy. Academic Press, 2021: 115-159.
[9] Tsutsuki K, Esaki I, Kuwatsuka S. CuO-oxidation products of peat as a key to the analysis of the paleo-environmental changes in a wetland[J]. Soil Science and Plant Nutrition, 1994, 40(1): 107-116. doi: 10.1080/00380768.1994.10414283
[10] Meuzelaar H L C, Haider K, Nagar B R, et al. Comparative studies of pyrolysis-mass spectra of melanins, model phenolic polymers, and humic acids[J]. Geoderma, 1977, 17(3): 239-252. doi: 10.1016/0016-7061(77)90054-4
[11] Bracewell J M, Robertson G W, Williams B L. Pyrolysis-mass spectrometry studies of humification in a peat and a peaty podzol[J]. Journal of Analytical & Applied Pyrolysis, 1980, 2(1): 53-62.
[12] Page D W. Characterisation of organic matter in sediment from Corin Reservoir, Australia[J]. Journal of Analytical and Applied Pyrolysis, 2003, 70(2): 169-183. doi: 10.1016/S0165-2370(02)00130-4
[13] Kumar M, Boski T, Lima-Filho F P, et al. Environmental changes recorded in the Holocene sedimentary infill of a tropical estuary[J]. Quaternary International, 2018, 476: 34-45. doi: 10.1016/j.quaint.2018.03.006
[14] Saiz-Jimenez C, de Leeuw J W. Lignin pyrolysis products: Their structures and their significance as biomarkers[J]. Organic Geochemistry, 1986, 10(4-6): 869-876. doi: 10.1016/S0146-6380(86)80024-9
[15] Tinoco P, Almendros G, Gonzalez-Vila F J. Impact of the vegetation on the lignin pyrolytic signature of soil humic acids from Mediterranean soils[J]. Journal of Analytical and Applied Pyrolysis, 2002, 64(2): 407-420. doi: 10.1016/S0165-2370(02)00041-4
[16] Carr A S, Boom A, Chase B M, et al. Molecular fingerprinting of wetland organic matter using pyrolysis-GC/MS: An example from the southern cape coastline of South Africa[J]. Journal of Paleolimnology, 2010, 44(4): 947-961. doi: 10.1007/s10933-010-9466-9
[17] Ohra-Aho T, Tenkanen M, Tamminen T. Direct analysis of lignin and lignin-like components from softwood kraft pulp by Py-GC/MS techniques[J]. Journal of Analytical and Applied Pyrolysis, 2005, 74(1-2): 123-128. doi: 10.1016/j.jaap.2004.11.010
[18] van der Kaaden A, Boon J J, Haverkamp J. The analytical pyrolysis of carbohydrates. 2-Differentiation of homopolyhexoses according to their linkage type, by pyrolysis-mass spectrometry and pyrolysis-gas chromatography/mass spectrometry[J]. Biomedical Mass Spectrometry, 1984, 11(9): 486-492. doi: 10.1002/bms.1200110910
[19] Ninnes S, Tolu J, Meyer-Jacob C, et al. Investigating molecular changes in organic matter composition in two Holocene lake-Sediment records from central Sweden using pyrolysis-GC/MS[J]. Journal of Geophysical Research: Biogeosciences, 2017, 122(6): 1423-1438. doi: 10.1002/2016JG003715
[20] Melenevskii V, Leonova G, Bobrov V, et al. Transformation of organic matter in the Holocene sediments of Lake Ochki (South Baikal region): Evidence from pyrolysis data[J]. Geochemistry International, 2015, 53(10): 903-921. doi: 10.1134/S0016702915080054
[21] Carr A S, Boom A, Chase B M, et al. Holocene sea level and environmental change on the west coast of South Africa: Evidence from plant biomarkers, stable isotopes and pollen[J]. Journal of Paleolimnology, 2015, 53(4): 415-432. doi: 10.1007/s10933-015-9833-7
[22] Tolu J, Gerber L, Boily J F, et al. High-throughput characterization of sediment organic matter by pyrolysis-gas chromatography/mass spectrometry and multivariate curve resolution: A promising analytical tool in (paleo)limnology[J]. Analytica Chimica Acta, 2015, 880: 93-102. doi: 10.1016/j.aca.2015.03.043
[23] Xie M M, Sun Q, Dong H W, et al. n-Alkanes and compound carbon isotope records from Lake Yiheshariwusu in the Hulun Buir sandy land, northeastern China[J]. The Holocene, 2020, 30(10): 1451-1461. doi: 10.1177/0959683620932968
[24] Schellekens J, Buurman P. n-Alkane distributions as palaeoclimatic proxies in ombrotrophic peat: The role of decomposition and dominant vegetation[J]. Geoderma, 2011, 164(3-4): 112-121. doi: 10.1016/j.geoderma.2011.05.012
[25] Shang W Y, Wang S X, Ling Y, et al. Holocene pyrolytic nitrogen compounds by using pyrolysis-GC/MS and its paleoclimatic implication from Jinchuan peatbog, NE China[J]. Quaternary International, 2022, 616: 109-119. doi: 10.1016/j.quaint.2021.10.006
[26] Kebelmann K, Hornung A, Karsten U, et al. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components[J]. Biomass and Bioenergy, 2013, 49: 38-48. doi: 10.1016/j.biombioe.2012.12.006
[27] Lara-Gonzalo A, Kruge M A, Lores I, et al. Pyrolysis GC-MS for the rapid environmental forensic screening of contaminated brownfield soil[J]. Organic Geochemistry, 2015, 87: 9-20. doi: 10.1016/j.orggeochem.2015.06.012
[28] Moldoveanu S C. Pyrolysis GC/MS, present and future (recent past and present needs)[J]. Journal of Microcolumn Separations, 2001, 13: 102-125. doi: 10.1002/mcs.1028
[29] Schellekens J, Bradley J A, Kuyper T W, et al. The use of plant-specific pyrolysis products as biomarkers in peat deposits[J]. Quaternary Science Reviews, 2015, 123: 254-264. doi: 10.1016/j.quascirev.2015.06.028
[30] Gupta N S. Molecular preservation of Eurypterids[M]//Biopolymers: A molecular paleontology approach. Dordrecht: Springer Netherlands, 2014: 119-134.
[31] Lu X, Ma S, Chen Y, et al. Squalene found in alpine grassland soils under a harsh environment in the Xizang Plateau, China[J]. Biomolecules, 2018, 8(4): 154-166. doi: 10.3390/biom8040154
[32] Piñeiro-Juncal N, Kaal J, Moreira J C F, et al. Cover loss in a seagrass Posidonia oceanica meadow accelerates soil organic matter turnover and alters soil prokaryotic communities[J]. Organic Geochemistry, 2021, 151: 104-140.
[33] Zhang Z, Wang J J, Lyu X, et al. Impacts of land use change on soil organic matter chemistry in the Everglades, Florida-A characterization with pyrolysis-gas chromatography-mass spectrometry[J]. Geoderma, 2019, 338: 393-400. doi: 10.1016/j.geoderma.2018.12.041
[34] Zhu R, Versteegh G J M, Hinrichs K U. Detection of microbial biomass in subseafloor sediment by pyrolysis-GC/MS[J]. Journal of Analytical and Applied Pyrolysis, 2016, 118: 175-180. doi: 10.1016/j.jaap.2016.02.002
[35] Zúñiga D, Kaal J, Villacieros-Robineau N, et al. Tracing sinking organic matter sources in the NW Iberian upwelling system (NE Atlantic Ocean): Comparison between elemental, isotopic and molecular indicators[J]. Journal of Analytical and Applied Pyrolysis, 2019, 139: 114-122. doi: 10.1016/j.jaap.2019.01.016
[36] 尚文郁, 孙静轶, 谢曼曼, 等. 基于Py-GC/MS的沙漠湖泊直链脂肪族化合物分析及古气候应用初探[J]. 岩矿测试, 2022, 41(5): 836-848. doi: 10.15898/j.cnki.11-2131/td.202201120009 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202201120009
Shang W Y, Sun J Y, Xie M M, et al. Py-GC/MS analysis method for aliphatic biomarker in desert lake sediment and its application in paleoclimatic study[J]. Rock and Mineral Analysis, 2022, 41(5): 836-848. doi: 10.15898/j.cnki.11-2131/td.202201120009 http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202201120009
[37] 丛浦珠, 苏克曼. 分析化学手册: 质谱分析(第九分册)[M]. 北京: 化学工业出版社, 2000.
Cong P Z, Su K M. Handbook of analytical chemistry: Mass spectrometry (Chapter 9)[M]. Beijing: Chemical Industry Press, 2000.
[38] Zhang J, Liu J, Liu R L. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate[J]. Bioresource Technology, 2015, 176: 288-291. doi: 10.1016/j.biortech.2014.11.011
[39] Garcia-Perez M, Wang X S, Shen J, et al. Fast pyrolysis of Oil Mallee Woody biomass: Effect of temperature on the yield and quality of pyrolysis products[J]. Industrial & Engineering Chemistry Research, 2008, 47(6): 1846-1854.
[40] Zhang H, Liao W, Zhou X, et al. Coeffect of pyrolysis temperature and potassium phosphate impregnation on characteristics, stability, and adsorption mechanism of phosphorus-enriched biochar[J]. Bioresource Technology, 2022, 344: 126273. doi: 10.1016/j.biortech.2021.126273
[41] Singh B P, Cowie A L, Smernik R J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature[J]. Environmental Science & Technology, 2012, 46(21): 11770-11778.
[42] Cui D, Li J, Zhang X, et al. Pyrolysis temperature effect on compositions of basic nitrogen species in Huadian shale oil using positive-ion ESI FT-ICR MS and GC-NCD[J]. Journal of Analytical and Applied Pyrolysis, 2021, 153: 104980. doi: 10.1016/j.jaap.2020.104980
[43] Kruge M A, Permanyer A. Application of pyrolysis-GC/MS for rapid assessment of organic contamination in sediments from Barcelona harbor[J]. Organic Geochemistry, 2004, 35(11): 1395-1408.
[44] Kaal J, Cortizas A M, Rydberg J, et al. Seasonal changes in molecular composition of organic matter in lake sediment trap material from Nylandssj n, Sweden[J]. Organic Geochemistry, 2015, 83-84: 253-262. doi: 10.1016/j.orggeochem.2015.04.005
[45] Fabbri D, Sangiorgi F, Vassura I. Pyrolysis-GC-MS to trace terrigenous organic matter in marine sediments: A comparison between pyrolytic and lipid markers in the Adriatic Sea[J]. Analytica Chimica Acta, 2005, 530(2): 253-261. doi: 10.1016/j.aca.2004.09.020
[46] Sigleo A C, Hoering T C, Helz G R. Composition of estuarine colloidal material: Organic components[J]. Geochimica et Cosmochimica Acta, 1982, 46(9): 1619-1626. doi: 10.1016/0016-7037(82)90318-0
[47] Kaal J. Analytical pyrolysis in marine environments revisited[J]. Analytical Pyrolysis Letters, 2019.
[48] Kaal J, Lavery P S, Martínez Cortizas A, et al. Recon-struction of 7500 years of coastal environmental change impacting seagrass ecosystem dynamics in Oyster Harbour (SW Australia)[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2020, 558: 109953. doi: 10.1016/j.palaeo.2020.109953
[49] Kaal J, Martinez Cortizas A, Mateo M A, et al. Deciphe-ring organic matter sources and ecological shifts in blue carbon ecosystems based on molecular fingerprinting[J]. Science of the Total Environment, 2020, 742: 140554. doi: 10.1016/j.scitotenv.2020.140554
[50] Boateng A A, Hicks K B, Vogel K P. Pyrolysis of switchgrass (Panicum virgatum) harvested at several stages of maturity[J]. Journal of Analytical & Applied Pyrolysis, 2006, 75(2): 55-64.
[51] Faure P, Jeanneau L, Lannuzel F. Analysis of organic matter by flash pyrolysis-gas chromatography-mass spectrometry in the presence of Na-smectite: When clay minerals lead to identical molecular signature[J]. Organic Geochemistry, 2006, 37(12): 1900-1912. doi: 10.1016/j.orggeochem.2006.09.008
[52] Zhang J, Wu C, Hou W, et al. Biological calcium carbonate with a unique organic-inorganic composite structure to enhance biochar stability[J]. Environmental Science: Processes & Impacts, 2021, 23(11): 1747-1758.
[53] Chmiel H E, Niggemann J, Kokic J, et al. Uncoupled organic matter burial and quality in boreal lake sediments over the Holocene[J]. Journal of Geophysical Research: Biogeosciences, 2015, 120(9): 1751-1763. doi: 10.1002/2015JG002987
[54] Moingt M, Lucotte M, Paquet S, et al. Deciphering the impact of land-uses on terrestrial organic matter and mercury inputs to large boreal lakes of central Québec using lignin biomarkers[J]. Applied Geochemistry, 2014, 41: 34-48. doi: 10.1016/j.apgeochem.2013.11.008
[55] Opsahl S, Benner R. Early diagenesis of vascular plant tissues: Lignin and cutin decomposition and biogeo-chemical implications[J]. Geochimica et Cosmochimica Acta, 1995, 59(23): 4889-4904. doi: 10.1016/0016-7037(95)00348-7
[56] Dittmar T, Lara R J. Molecular evidence for lignin degradation in sulfate-reducing mangrove sediments (Amazônia, Brazil)[J]. Geochimica et Cosmochimica Acta, 2001, 65(9): 1417-1428. doi: 10.1016/S0016-7037(00)00619-0
[57] Sanjurjo-Sánchez J, Kaal J, Fenollós J L M. Organic matter from bevelled rim bowls of the middle Euphrates: Results from molecular characterization using pyrolysis-GC-MS[J]. Microchemical Journal, 2018, 141: 1-6. doi: 10.1016/j.microc.2018.05.001
[58] Kaal J, Cortizas A M, Biester H. Downstream changes in molecular composition of DOM along a headwater stream in the Harz Mountains (central Germany) as determined by FTIR, pyrolysis-GC-MS and THM-GC-MS[J]. Journal of Analytical and Applied Pyrolysis, 2017, 126: 50-61. doi: 10.1016/j.jaap.2017.06.025
[59] 杨丽阳, 吴莹, 黄俊华, 等. 大九湖泥炭柱样的木质素特征[J]. 地球化学, 2009, 38(2): 133-139. doi: 10.3321/j.issn:0379-1726.2009.02.004
Yang L Y, Wu Y, Huang J H, et al. Lignin characteristics in a peat core of Lake Dajiu[J]. Geochimica, 2009, 38(2): 133-139. doi: 10.3321/j.issn:0379-1726.2009.02.004
[60] 刘月, 王敏, 张婷, 等. 杭州湾外泥质区柱状沉积物中木质素的分布特征及其环境指示意义[J]. 海洋环境科学, 2017, 36(1): 8-14. doi: 10.13634/j.cnki.mes.2017.01.002
Liu Y, Wang M, Zhang T, et al. Distribution characteristics of lignin in sediment cores from the mud area off Hangzhou Bay and the implication for regional sedimentary environment[J]. Chinese Journal of Marine Environmental Science, 2017, 36(1): 8-14. doi: 10.13634/j.cnki.mes.2017.01.002
[61] Tuomela M, Hatakka A. Oxidative fungal enzymes for bioremediation[M]//Comprehensive biotechnology (The second edition). Burlington: Academic Press, 2011.
[62] Otsuka Y, Sonoki T, Ikeda S, et al. Detection and characterization of a novel extracellular fungal enzyme that catalyzes the specific and hydrolytic cleavage of lignin guaiacylglycerol β-aryl ether linkages[J]. European Journal of Biochemistry, 2003, 270(11): 2353-2362. doi: 10.1046/j.1432-1033.2003.03545.x
[63] Wong D W S. Structure and action mechanism of ligninolytic enzymes[J]. Applied Biochemistry and Biotechnology, 2009, 157(2): 174-209. doi: 10.1007/s12010-008-8279-z
[64] Boerjan W, Ralph J, Baucher M. Lignin Biosynthesis[J]. Annual Review of Plant Biology, 2003, 54(1): 519-546. doi: 10.1146/annurev.arplant.54.031902.134938
[65] Philben M, Ziegler S E, Edwards K A, et al. Soil organic nitrogen cycling increases with temperature and precipitation along a boreal forest latitudinal transect[J]. Biogeochemistry, 2016, 127(2): 397-410.
[66] Hawkes C V, Kivlin S N, Rocca J D, et al. Fungal community responses to precipitation[J]. Global Change Biology, 2015, 17(4): 1637-1645.
[67] Xiao J, Chang Z, Wen R, et al. Holocene weak monsoon intervals indicated by low lake levels at Hulun Lake in the monsoonal margin region of northeastern Inner Mongolia, China[J]. Holocene, 2009, 19(6): 899-908. doi: 10.1177/0959683609336574
[68] Wen R, Xiao J, Chang Z, et al. Holocene precipitation and temperature variations in the East Asian monsoonal margin from pollen data from Hulun Lake in northeastern Inner Mongolia, China[J]. Boreas, 2010, 39(2): 262-272. doi: 10.1111/j.1502-3885.2009.00125.x
[69] 曾琳, 鹿化煜, 弋双文, 等. 末次盛冰期和全新世大暖期呼伦贝尔沙地的环境变化[J]. 第四纪研究, 2013, 33(2): 243-251. doi: 10.3969/j.issn.1001-7410.2013.02.05
Lin Z, Lu H Y, Yi S W, et al. Environmental changes of Hulun Buir dunefield in northeastern China during the last Glacial maximum and Holocene optimum[J]. Quaternary Sciences, 2013, 33(2): 243-251. doi: 10.3969/j.issn.1001-7410.2013.02.05
[70] Li S H, Sun J. Optical dating of Holocene dune sands from the Hulun Buir Desert, northeastern China[J]. Holocene, 2006, 16(3): 457-462. doi: 10.1191/0959683606hl942rr
[71] 温锐林, 肖举乐, 常志刚, 等. 全新世呼伦湖区植被和气候变化的孢粉记录[J]. 第四纪研究, 2010, 30(6): 1105-1115. doi: 10.3969/j.issn.1001-7410.2010.06.05
Wen R L, Xiao J L, Chang Z G, et al. Holocene vegetation and climate changes reflected by the pollen record of Hulun Lake, north eastern Inner Mongolia[J]. Quaternary Sciences, 2010, 30(6): 1105-1115. doi: 10.3969/j.issn.1001-7410.2010.06.05
[72] Na L, Yu L, Guang B, et al. Drought reconstruction in eastern Hulun Buir steppe, China and its linkages to the sea surface temperatures in the Pacific Ocean[J]. Journal of Asian Earth Sciences, 2016, 115: 298-307. doi: 10.1016/j.jseaes.2015.10.009
[73] 张风菊, 薛滨, 姚书春. 中全新世以来呼伦湖沉积物碳埋藏及其影响因素分析[J]. 湖泊科学, 2018, 30(1): 234-244. https://www.cnki.com.cn/Article/CJFDTOTAL-FLKX201801024.htm
Zhang F J, Xue B, Yao S C. Organic carbon burial and its driving mechanism in the sediment of Lake Hulun, northeastern Inner Mongolia, since the mid-Holocene[J]. Journal of Lake Sciences, 2018, 30(1): 234-244. https://www.cnki.com.cn/Article/CJFDTOTAL-FLKX201801024.htm
[74] 董浩伟, 赵佳玉, 曾凡刚, 等. 柱色谱分离-分子筛络合洗脱过程中正构烷烃单体碳同位素分馏研究[J]. 岩矿测试, 2021, 40(3): 349-357. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202005030063
Dong H W, Zhao J Y, Zeng F G, et al. Study on specific carbon isotope fractionation of n-alkanes during column chromatography separation-molecular sieve complexation adsorption[J]. Rock and Mineral Analysis, 2021, 40(3): 349-357. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.202005030063
[75] 王宁, 朱庆增, 谢曼曼, 等. 尿素络合法分离-气相色谱/同位素质谱法分析土壤和植物中低含量(ppm级)正构烷烃的碳同位素[J]. 岩矿测试, 2015, 34(4): 471-479. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.2015.04.016
Wang N, Zhu Q Z, Xie M M, et al. An improved urea adduction method for analyzing carbon isotope of ppm-level n-alkanes in soil and plant samples[J]. Rock and Mineral Analysis, 2015, 34(4): 471-479. http://www.ykcs.ac.cn/cn/article/doi/10.15898/j.cnki.11-2131/td.2015.04.016
[76] Routson C C, McKay N P, Kaufman D S, et al. Mid-latitude net precipitation decreased with Arctic warming during the Holocene[J]. Nature, 2019, 568(7750): 83-87. doi: 10.1038/s41586-019-1060-3
-




 下载:
下载: