Preparation and Application of Biochar-Chitosan Magnetic Composite Adsorbent for Removal of Lead and Copper from Groundwater
-
摘要:
壳聚糖作为天然多糖有机物,具有对环境友好的特性,其含有的大量含氮官能团可吸附水中的金属离子。但壳聚糖类吸附剂在酸性条件下适应性差,实际使用过程中需要调节pH值,因此增加了运行成本。本文选用农林废弃物花生壳(CC)和玉米芯(PS)制备生物炭,与壳聚糖进行结合,并引入磁性因子Fe3O4,制备了花生壳生物炭-壳聚糖磁性复合吸附剂(PSC)和玉米芯生物炭-壳聚糖磁性复合吸附剂(CCC),并研究这两种吸附剂对水中Pb2+和Cu2+的吸附性能,同时利用实际含多种金属离子的地下水对所制备的材料进行实验,以评估其实际应用潜能。比表面积仪(BET)分析表征显示,CCC相比PSC的比表面积和平均孔径更大,两种吸附剂在pH 4~7范围内均表现出稳定的吸附性能。循环5个周期后,两种吸附剂仍对Pb2+和Cu2+的去除率保持在85%以上,表现出良好的循环利用性能。CCC对Pb2+和Cu2+的最大吸附容量分别为169.10mg/g和18.69mg/g,均大于PSC的最大吸附容量。同时,CCC可有效去除含重金属地下水中的多种金属离子。在处理实际含Pb2+和Cu2+的废水时可优先选择CCC材料作为吸附剂。吸附动力学实验结果表明,两种材料对Pb2+的吸附以物理吸附为主,对Cu2+的吸附以化学吸附为主。pH值影响实验和X射线光电子能谱(XPS)表征结果说明两种材料主要通过静电吸引和含氮官能团与金属离子的螯合作用去除Pb2+和Cu2+。本文使用农林废弃物制备生物炭降低了成本,引入的磁性因子方便了脱附过程,生物炭-壳聚糖磁性复合材料的制备方法有效地改善了壳聚糖类材料在酸性条件下的适应性,所制备的材料是一种去除地下水中Pb2+和Cu2+污染的有效潜在吸附剂。
Abstract:BACKGROUND Wastewater containing heavy metals produced by mining, beneficiation, smelting, forging, processing, transportation, and other industries that has been improperly disposed of, leads to heavy metals entering and polluting the groundwater environment. Heavy metals can be enriched in the human body and participate in the biological cycle. Long-term accumulation of heavy metals in the human body will bring carcinogenic, teratogenic, and mutagenic risks. Adsorption has been of wide concern in the treatment of heavy metals pollution in water, due to the advantages of low operation cost, simple engineering operation and low secondary pollution. Chitosan as a natural organic polysaccharide organic matter, has the characteristics of being environmentally friendly. It contains many nitrogen-containing functional groups that can adsorb metal ions in water. However, the adaptability of chitosan adsorbents to acidic conditions is poor, so the pH value needs to be adjusted in the actual process, which increases the operating cost. Combining biochar with chitosan can not only improve the adsorption capacity of chitosan, but also improve the separation performance of biochar. However, most of the research on chitosan modified biochar concentrate on the single biochar. There are few studies on the modification of biochar from different sources by chitosan, and the interaction between chitosan and biochar is not clear.
OBJECTIVES The aim of this study was to prepare peanut shell biochar-chitosan magnetic composite adsorbent (PSC) and corn cob biochar-chitosan magnetic composite adsorbent (CCC), and to investigate the Pb2+/Cu2+ adsorption properties and mechanisms on PSC and CCC.
METHODS Scanning electron microscope was used to analyze the microstructure of the material, and the material samples were treated with gold spray before photographing. The specific surface area and pore volume of the material were determined using a specific surface area analyzer, and the material was adsorption-desorption tested with nitrogen at -196℃. X-ray diffraction was used to analyze the crystal structure of materials, and the Cu Kα source was used to scan in the range of 10°-80° (2θ). X-ray photoelectron spectrometer was used to analyze the changes of functional groups on the surface of the material, and the radiation (225W, 15mA, 15kV) was carried out by monochromatic Al-Kα. Metal ion content in solution was measured by inductively coupled plasma-optical emission spectrometry. The adsorption experiments were carried out in a 50mL conical flask and a constant temperature shaker. The oscillation frequency was 150r/min and the reaction time was 12h. After the reaction, the concentrations of Pb2+ and Cu2+ in the solution were determined. Two parallel samples were set up in each group. Different initial pH experiments were used to evaluate the adsorption performance of materials. Kinetic and isothermic models were used to evaluate the adsorption kinetic process of materials and predict the maximum adsorption capacity of materials. Recycling experiments and actual mine groundwater adsorption experiments were used to evaluate the practical application capacity of materials.
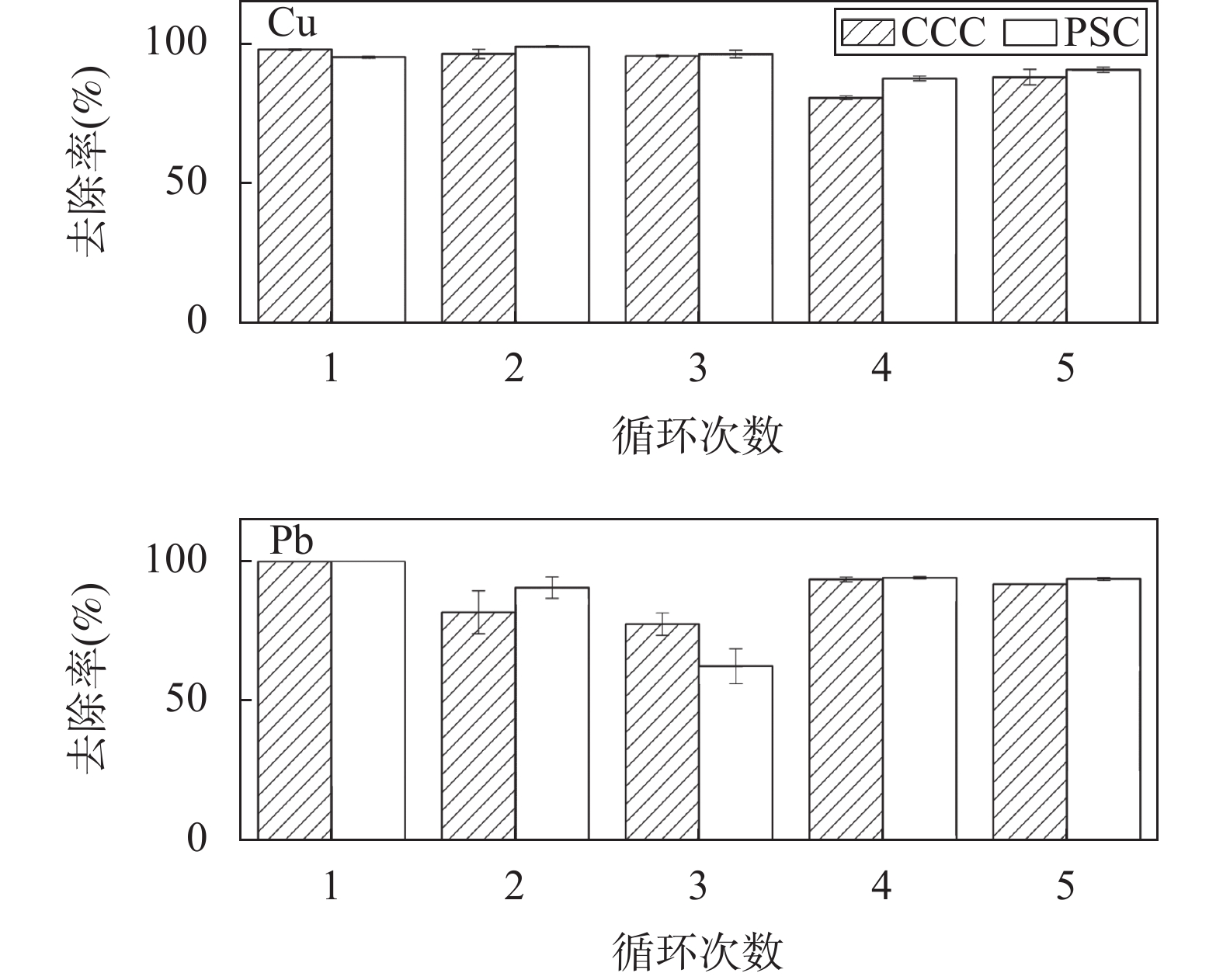
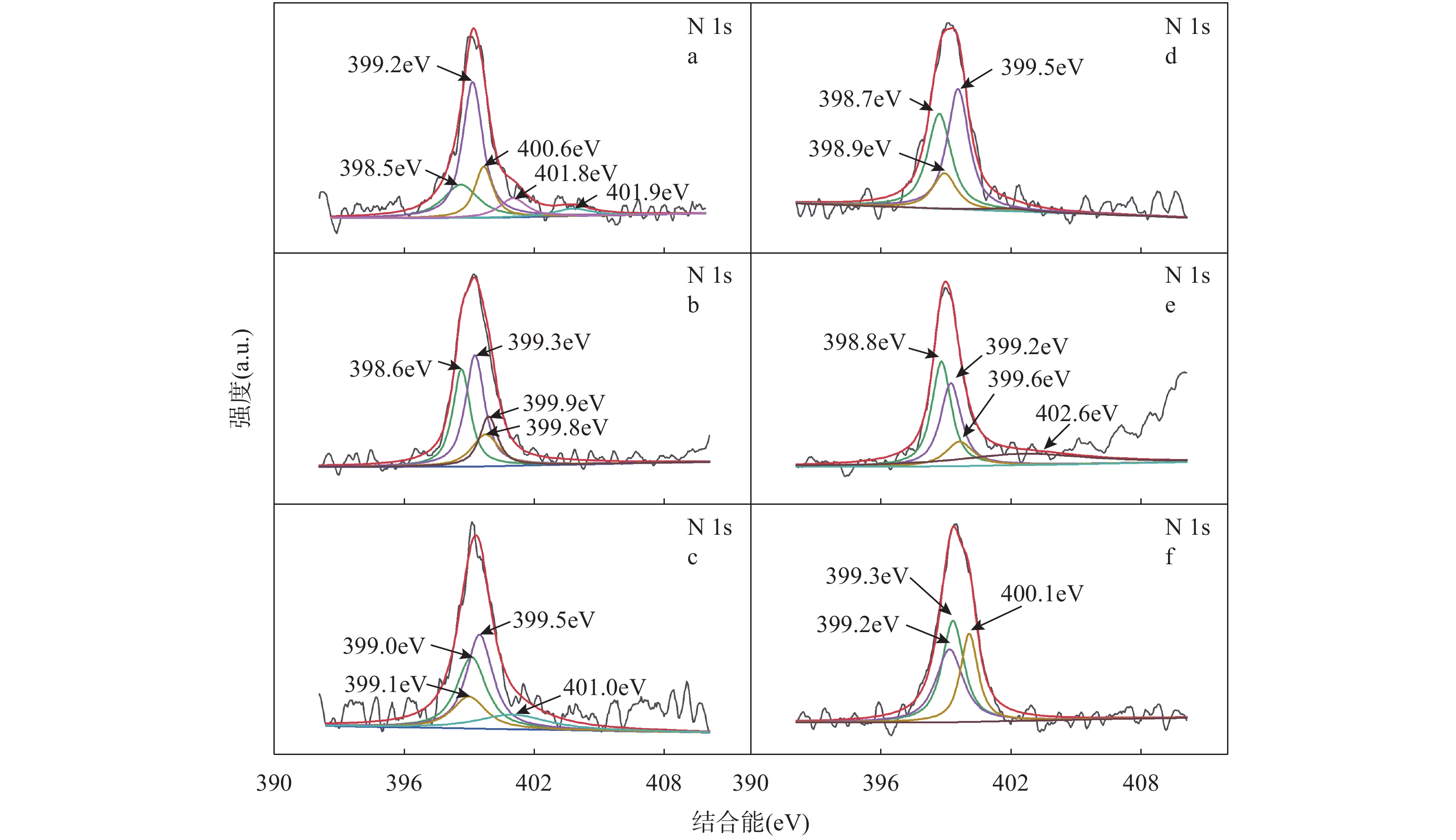
RESULTS SEM images of PS and CC showed that there were many pores on the surface of both types of carbon. There were more pores and bulges observed on the surface of CC than PS, indicating that CC had a larger contact area with pollutants than PS. The specific surface area (12.045m2/g) and mean pore diameter (3.614nm) of CC were larger than those of PS (specific surface area was 3.294m2/g and mean pore diameter was 3.067nm), which was consistent with the SEM results. The specific surface area (4.598m2/g) and average pore diameter (3.417nm) was 2.812nm), indicating that the primary structural properties of biochar affected the pore structure of the composites. SEM of CCC and PSC showed that chitosan and biochar were well combined. Compared with previous literature, the biochar-chitosan composite material in this study preserves the original structure of biochar to the greatest extent, and makes the chitosan evenly coated on the surface of biochar. The results BET showed that the primary structural properties of the biochar affected the physical properties of the modified materials. The XRD results showed that Fe3O4 was successfully embedded into the composite material. Three pH values (4, 7 and 10) were selected to evaluate the swelling properties of the materials. The results showed that the swelling ratios of the two materials were similar under the three pH conditions, and neither of them was more than 100.0%, which was due to the biochar as a carrier having a good supporting effect. Compared with chitosan/kiwifruit branch biochar adsorbent, the two adsorbents in this study showed relatively stable adsorption properties in the pH range of 4-7, indicating that the adsorbents had a wider range of pH value application. The effect of the initial pH on the adsorption was tested. As the initial pH value increased from 3 to 7, the adsorption capacity and removal efficiency of the material increased. The positive charge of the adsorbent surface also decreased, which reduced the electrostatic repulsion of Pb2+ and Cu2+ between the material surface and the solution, and increased the electrostatic attraction between the material surface and Pb2+ and Cu2+. The increasing electrostatic attraction was beneficial to the adsorption of Pb2+ and Cu2+. When PSC and CCC adsorbed Pb2+, the pseudo-first-order kinetic model could better describe the adsorption process than the pseudo-second-order kinetic model, indicating that physical adsorption played a dominant role in the adsorption of Pb2+ by PSC and CCC. Pseudo-second-order kinetics can better fit the Cu2+ adsorption by PSC and CCC than pseudo-second-order kinetic model, indicating that chemisorption dominates the adsorption process of Cu2+ by PSC and CCC. The Langmuir isotherm adsorption model can better describe the adsorption process of Pb2+ and Cu2+ than Freundlich isotherm adsorption model, indicating that the adsorption process is monolayer adsorption. The EDTA-2Na was used as the desorption agent of chitosan composite, the removal efficiencies of Pb2+ and Cu2+ were still above 85% after five cycles, which indicated that the two adsorbents in this study had excellent stability. PSC and CCC were the low-cost and effective adsorption materials. Groundwater of an acid mine was taken from a mining area in Dongshan District, Dafan Mountain, Anhui Province. The groundwater samples contained large amounts of metal ions, such as As, Ca, Cd, Cu, Fe, Mn, Na and Ni, the corresponding concentrations were 18.200g/L, 40.541mg/L, 3.800g/L, 13.300mg/L, 215.00mg/L, 510.00g/L, 81.694mg/L and 87.000g/L, respectively. 0.45μm filter membrane was used to remove particulate impurities from the water before adsorption. CCC was used to treat the heavy metal polluted groundwater, and the results showed that CCC has a good removal ability on a variety of metal ions in groundwater such as Cu2+, Cd2+, and Fe3+. The Cu2+ concentration in the treated water can reach the Grade IV water standard of the “Groundwater Quality Standard”. The pH results showed that the removal mechanisms of Pb2+ and Cu2+ by PSC and CCC mainly included electrostatic attraction. The functional groups of adsorbents before and after adsorption were analyzed through X-ray photoelectron spectroscopy (XPS). The results of XPS showed that complexation was another removal mechanism. The nitrogen (—NH) of pyrroles, amino of chitosan (—NH2), and C=N were the main functional groups, which were responsible for the complexation with Pb2+ and Cu2+.
CONCLUSIONS The preparation method of the materials in this study can effectively improve the adaptability of chitosan materials under different pH conditions. The adsorbents developed in this study can effectively remove heavy metals from groundwater and have a good application potential.
-
Key words:
- biochar /
- chitosan /
- adsorption /
- lead /
- copper /
- N-containing functional groups; X-ray photoelectron spectroscopy
-

-
表 1 PSC和CCC吸附Pb2+、Cu2+动力学模型拟合参数以及PSC和CCC吸附Pb2+、Cu2+等温线模型拟合参数
Table 1. Fitting parameters of kinetic models for Pb2+ and Cu2+ adsorption by PSC and CCC, and the fitting parameters of PSC and CCC adsorption of Pb2+ and Cu2+ isotherm models.
金属离子 制备
材料伪一级动力学模型 伪二级动力学模型 K1 R2 χ2 k2 R2 χ2 Pb2+ PSC 0.0375 0.998 0.042 0.0062 0.936 0.114 CCC 0.0045 0.999 0.044 0.0004 0.986 0.121 Cu2+ PSC 0.0072 0.996 0.049 0.0009 0.997 0.135 CCC 0.0037 0.988 0.082 0.0021 0.997 0.056 金属离子 制备
材料Langmuir模型 Freundlich模型 qm KL R2 χ2 KF 1/n R2 χ2 Pb2+ PSC 189.09 0.0017 0.999 0.702 0.766 0.749 0.992 8.460 CCC 86.72 0.0036 0.998 1.420 1.220 0.622 0.984 9.706 Cu2+ PSC 18.69 0.0123 0.981 0.973 1.277 0.419 0.952 2.372 CCC 14.34 0.0197 0.979 0.667 2.72 0.368 0.928 0.375 表 2 PSC和CCC吸附Pb和Cu的性能与其他材料对比
Table 2. Comparison of the adsorption capacities of PSC and CCC with other materials.
-
[1] Feng Z Y,Chen N,Liu T,et al. KHCO3 activated biochar supporting MgO for Pb(Ⅱ) and Cd(Ⅱ) adsorption from water:Experimental study and DFT calculation analysis[J]. Journal of Hazardous Materials, 2022, 426:128059. doi: 10.1016/j.jhazmat.2021.128059
[2] Yang G X,Jiang H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater[J]. Water Research, 2014, 48:396−405. doi: 10.1016/j.watres.2013.09.050
[3] Deng J Q,Liu Y G,Liu S B,et al. Competitive adsorption of Pb(Ⅱ),Cd(Ⅱ) and Cu(Ⅱ) onto chitosan-pyromellitic dianhydride modified biochar[J]. Journal of Colloid and Interface Science, 2017, 506:355−364. doi: 10.1016/j.jcis.2017.07.069
[4] Jafarzadeh N,Heidari K,Meshkinian A,et al. Non-carcinogenic risk assessment of exposure to heavy metals in underground water resources in Saraven,Iran:Spatial distribution,monte-carlo simulation,sensitive analysis[J]. Environmental Research, 2022, 204:112002. doi: 10.1016/j.envres.2021.112002
[5] Wang Q,Zhao Y,Zhai S,et al. Application of different redox mediators induced bio-promoters to accelerate the recovery of denitrification and denitrifying functional microorganisms inhibited by transient Cr(Ⅵ) shock[J]. Journal of Hazardous Materials, 2021, 420:126664. doi: 10.1016/j.jhazmat.2021.126664
[6] Conceição F T,Silva M S G,Menegário A A,et al. Precipitation as the main mechanism for Cd(Ⅱ),Pb(Ⅱ) and Zn(Ⅱ) removal from aqueous solutions using natural and activated forms of red mud[J]. Environmental Advances, 2021, 4:100056. doi: 10.1016/j.envadv.2021.100056
[7] Chen Y,Yang X. Molecular simulation of layered GO membranes with amorphous structure for heavy metal ions separation[J]. Journal of Membrane Science, 2022, 660:120863. doi: 10.1016/j.memsci.2022.120863
[8] 崔婷,叶欣,朱霞萍,等. 土壤铁锰氧化物形态测定及吸附Sb(Ⅲ)的主控因子研究[J]. 岩矿测试,2023,42(1):167−176.
Cui T,Ye X,Zhu X P,et,al. Determination of various forms of iron and manganese oxides and the main controlling factors of absorption of Sb(Ⅲ) in soil[J]. Rock and Mineral Analysis, 2023, 42(1):167−176.
[9] Jyoti D,Sinha R,Faggio C. Advances in biological methods for the sequestration of heavy metals from water bodies:A review[J]. Environmental Toxicology and Pharmacology, 2022, 94:103927. doi: 10.1016/j.etap.2022.103927
[10] Chen Q,Luo Z,Hills C,et al. Precipitation of heavy metals from wastewater using simulated flue gas:Sequent additions of fly ash,lime and carbon dioxide[J]. Water Research, 2009, 43:2605−2614. doi: 10.1016/j.watres.2009.03.007
[11] Luo J,Maier R M,Yu D,et al. Crittenden,double-network hydrogel:A potential practical adsorbent for critical metals extraction and recovery from water[J]. Environmental Science & Technology, 2022, 56:4715−4717.
[12] Wu J W,Wang T,Wang J W,et al. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar:Enhanced the ion exchange and precipitation capacity[J]. Science of the Total Environment, 2021, 754:142150. doi: 10.1016/j.scitotenv.2020.142150
[13] 聂雪. 改性壳聚糖金属螯合物的合成及电催化性能研究[D]. 长沙: 中南大学, 2005.
Nie X. Synthesis and electrocatalytic properties of modified chitosan metal chelates[D]. Changsha: Central South University, 2005.
[14] Jiang B,Lin Y,Mbog J C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics[J]. Bioresource Technology, 2018, 270:603−611. doi: 10.1016/j.biortech.2018.08.022
[15] Zhao C,Liu G,Sun N,et al. Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization[J]. Chemical Engineering Journal, 2018, 334:1270−1280. doi: 10.1016/j.cej.2017.11.069
[16] Nazari S,Rahimi G,Khadem A. Effectiveness of native and citric acid-enriched biochar of chickpea straw in Cd and Pb sorption in an acidic soil[J]. Journal of Environmental Chemical Engineering, 2019, 7:103064. doi: 10.1016/j.jece.2019.103064
[17] Wang F,Jin L,Guo C,et al. Enhanced heavy metals sorption by modified biochars derived from pig manure[J]. Science of the Total Environment, 2021, 786:147595. doi: 10.1016/j.scitotenv.2021.147595
[18] Song J Y,Messele S A,Meng L J,et al. Adsorption of metals from oil sands process water (OSPW) under natural pH by sludge-based biochar/chitosan composite[J]. Water Research, 2021, 194:116930. doi: 10.1016/j.watres.2021.116930
[19] Feng Z Y,Feng C P,Chen N,et al. Preparation of composite hydrogel with high mechanical strength and reusability for removal of Cu(Ⅱ) and Pb(Ⅱ) from water[J]. Separation and Purification Technology, 2022, 300:121894. doi: 10.1016/j.seppur.2022.121894
[20] Chang Z,Tian L,Dong J,Chen Q,et al. The molecular markers provide complementary information for biochar characterization before and after HNO3/H2SO4 oxidation[J]. Chemosphere, 2022, 301:134422. doi: 10.1016/j.chemosphere.2022.134422
[21] Du H,Xi C,Tang B,et al. Performance and mechanisms of NaOH and ball-milling co-modified biochar for enhanced the removal of Cd2+ in synthetic water:A combined experimental and DFT study[J]. Arabian Journal of Chemistry, 2022, 15:103817. doi: 10.1016/j.arabjc.2022.103817
[22] Yin G,Tao L,Chen X,et al. Quantitative analysis on the mechanism of Cd2+ removal by MgCl2-modified biochar in aqueous solutions[J]. Journal of Hazardous Materials, 2021, 420:126487. doi: 10.1016/j.jhazmat.2021.126487
[23] Gao N,Du W,Zhang M G,et al. Chitosan-modified biochar:Preparation,modifications,mechanisms and applications[J]. International Journal of Biological Macromolecules, 2022, 209:31−49. doi: 10.1016/j.ijbiomac.2022.04.006
[24] Mallik A K,Kabir S F,Sakib M N,et al. Cu(Ⅱ) removal from wastewater using chitosan-based adsorbents:A review[J]. Journal of Environmental Chemical Engineering, 2022, 10:108048. doi: 10.1016/j.jece.2022.108048
[25] Li Y,Zhou Y,Nie W,et al. Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles[J]. Journal of Porous Materials, 2015, 22:1383−1392. doi: 10.1007/s10934-015-0017-7
[26] Javanbakht V,Ghoreishi S M,Habibi N,et al. A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(Ⅱ) ions from aqueous solution[J]. Powder Technology, 2016, 302:372−383. doi: 10.1016/j.powtec.2016.08.069
[27] Charpentier T V J,Neville A,Lanigan J L,et al. Preparation of magnetic carboxymethylchitosan nanoparticles for adsorption of heavy metal ions[J]. ACS Omega, 2016, 1:77−83. doi: 10.1021/acsomega.6b00035
[28] 李满林. 氨基硫脲及氨基化壳聚糖衍生物的合成与应用研究[D]. 杨凌: 西北农林科技大学, 2014.
Li M L. Synthesis and application of thiourea and chitosan derivatives[D]. Yangling: Northwest A&F University, 2014.
[29] Xiang J,Lin Q,Cheng S,et al. Enhanced adsorption of Cd(Ⅱ) from aqueous solution by a magnesium oxide-rice husk biochar composite[J]. Environmental Science and Pollution Research, 2018, 25:14032−14042. doi: 10.1007/s11356-018-1594-1
[30] Zhou Y,Gao B,Fang J,et al. Sorption of heavy metals on chitosan-modified biochars and its biological effects[J]. Chemical Engineering Journal, 2013, 231:512−518. doi: 10.1016/j.cej.2013.07.036
[31] Xiang J,Lin Q,Yao X,et al. Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in-situ remediation of Cd-contaminated soil[J]. Environmental Research, 2021, 195:110650. doi: 10.1016/j.envres.2020.110650
[32] Xu L,Liu Y,Wang J,et al. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite:Kinetic,thermal dynamic and DFT studies[J]. Journal of Hazardous Materials, 2021, 404:124140. doi: 10.1016/j.jhazmat.2020.124140
[33] 赵子科,陈春亮,柯盛,等. 榴莲壳和不同炭材料对低汞溶液的吸附动力学[J]. 岩矿测试,2022,41(1):90−98. doi: 10.3969/j.issn.0254-5357.2022.1.ykcs202201009
Zhao Z K,Chen C L,Ke S,et al. Adsorption kinetics of durian shell and different carbon materials to low mercury solution[J]. Rock and Mineral Analysis, 2022, 41(1):90−98. doi: 10.3969/j.issn.0254-5357.2022.1.ykcs202201009
[34] 许芳,张利平,程先忠,等. 改性桑树叶吸附材料对废水中Cd(Ⅱ)的吸附性能研究[J]. 岩矿测试,2016,35(1):62−68.
Xu F,Zhang L P,Cheng X Z,et al. Study on the adsorption performance of modified mulberry leaf adsorbent for Cd(Ⅱ) in wastewater[J]. Rock and Mineral Analysis, 2016, 35(1):62−68.
[35] Karthik R,Meenakshi S. Removal of Pb(Ⅱ) and Cd(Ⅱ) ions from aqueous solution using polyaniline grafted chitosan[J]. Chemical Engineering Journal, 2015, 263:168−177. doi: 10.1016/j.cej.2014.11.015
[36] Bai R X,Zhang Y,Zhao Z G,et al. Rapid and highly selective removal of lead in simulated wastewater of rare-earth industry using diglycolamic-acid functionalized magnetic chitosan adsorbents[J]. Journal of Industrial and Engineering Chemistry, 2018, 59(25):416−424.
[37] Ren Y,He F,Peng H,et al. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent:Preparation,characterization,and application in heavy metal adsorption[J]. Chemical Engineering Journal, 2013, 226(12):300−311.
[38] Rozumová L,Zivotský O,Seidlerová J,et al. Magnetically modified peanut husks as an effective sorbent of heavy metals[J]. Journal of Environmental Chemical Engineering, 2016, 4(1):549−555. doi: 10.1016/j.jece.2015.10.039
[39] Luo X,Zeng J,Liu S,et al. An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system:Magnetic chitosan/cellulose microspheres[J]. Bioresource Technology, 2015, 194:403−406. doi: 10.1016/j.biortech.2015.07.044
[40] Culita D C,Simonescu C M,Dragne M,et al. Effect of surfactant concentration on textural,morphological and magnetic properties of CoFe2O4 nanoparticles and evaluation of their adsorptive capacity for Pb(Ⅱ) ions[J]. Ceramics International, 2015, 41(10):13553−13560. doi: 10.1016/j.ceramint.2015.07.150
[41] Ifthikar J,Jiao X,Ngambia A,et al. Facile one-pot synthesis of sustainable carboxymethyl chitosan-sewage sludge biochar for effective heavy metal chelation and regeneration[J]. Bioresource Technology, 2018, 262:22−31. doi: 10.1016/j.biortech.2018.04.053
[42] Afzal M Z,Sun X F,Liu J,et al. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads[J]. Science of the Total Environment, 2018, 639:560−569. doi: 10.1016/j.scitotenv.2018.05.129
[43] Zhou Y M,Fu S Y,Zhang L L,et al. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(Ⅱ)[J]. Carbohydrate Polymers, 2014, 101:75−82. doi: 10.1016/j.carbpol.2013.08.055
[44] 肖雪婷. Fe3O4-壳聚糖@生物炭的制备及处理油田采出水试验研究[D]. 沈阳: 沈阳建筑大学, 2020.
Xiao X T. Study on preparation of Fe3O4-chitosan@biochar and treatment of oilfield produced water[D]. Shenyang: Shenyang Jianzhu University, 2020.
[45] Tan Y,Wan X,Ni X,et al. Efficient removal of Cd(Ⅱ) from aqueous solution by chitosan modified kiwi branch biochar[J]. Chemosphere, 2022, 289:133251. doi: 10.1016/j.chemosphere.2021.133251
-




 下载:
下载: