Comparison and Optimization of Sr Isotope Analysis in Carbonate Rocks by Multiple-step Leaching Method
-
摘要:
海相沉积碳酸盐岩是记录海水信息的重要载体,其中碳酸盐岩的Sr同位素比值(87Sr/86Sr)可以反映大陆地壳和地幔对海水组成的相对贡献,其长期变化趋势可用于反演地球历史上的全球构造事件、风化速率变化和生物地球化学循环以及确定海相沉积地层年龄等。然而,现存古老地层中的碳酸盐岩常不同程度地保留有非碳酸盐组分,并受到后期蚀变影响,从而使碳酸盐岩全岩的Sr同位素组成不等同于原生碳酸盐组分的Sr同位素组成。为了获取可以反映当时的海水组成的原生碳酸盐组分,需要建立一种有效的浸提方法。本文基于已有的两种碳酸盐岩多步浸提方法,探讨了其适用性,并简化了预浸步骤、缩短了流程时间,明确了不同种类的碳酸盐岩的浸提方法。结果表明:碳酸盐纯度≥85%的灰岩适用于采用浸提液为1%乙酸的9步浸提法,目标步骤为L7~L9;纯度≥65%的白云岩样品适用于采用浸提液为0.25%~10%乙酸的14步浸提法,目标步骤为D13~D14,采用多接收电感耦合等离子体质谱仪(MC-ICP-MS)对纯化后的目标浸提液进行Sr同位素测试。通过此方法获得中国灰岩标准物质GBW03105a原生碳酸盐组分的87Sr/86Sr比值为0.708930±0.000015(n=12,2SD),与前人研究结果(0.708879±0.000013)相吻合;并报道了欧洲钢铁标准化委员会(ECISS)白云岩标准物质ECRM-782-1原生碳酸盐组分的87Sr/86Sr比值为0.707868±0.000034(n=12,2SD),为后续地球化学分析提供了方法和数据支持。
Abstract:BACKGROUND Marine sedimentary carbonate rock is an important carrier for recording seawater information. The Sr isotope composition (87Sr/86Sr) of carbonate rocks can reflect the relative contribution of the continental crust and mantle to the Sr isotope composition of seawater. The long-term variation trend of Sr isotope composition in geological history can be used to interpret global tectonic events, weathering rate changes, biogeochemical cycles, and determine the age of marine sedimentary strata. However, the carbonate rocks likely contain non-carbonate fractions to varying degrees, which lead to the whole rock Sr isotope composition being unequal to that of the primary carbonate fraction. In order to obtain the primary carbonate fraction that reflects the primitive seawater, an effective leaching method is required.
OBJECTIVES To identify experimental procedures and target leaching steps that can effectively extract representative primary carbonate fractions in carbonate rock samples of varying purity and variety.
METHODS Reference materials of dolostone and limestone (GBW03105a and ECRM-782-1) were selected to represent carbonate rock samples with high purity, and natural samples of limestone (C-3, purity: 85%) and dolostone (E-3, purity: 65%) were selected to represent samples with low purity. The leaching solution of all steps was measured for Ca and Mg contents by inductively coupled plasma-optical emission spectrometry (ICP-OES), for Sr, Mn and Al contents by inductively coupled plasma-mass spectrometry (ICP-MS). The Sr isotope was measured by multi-collector inductively coupled plasma-mass spectrometry (MC-ICP-MS) after purification of the leaching solution. Through the utilization of various indicators, such as Sr/Ca and Mn/Sr, the targeted leaching steps were ascertained.
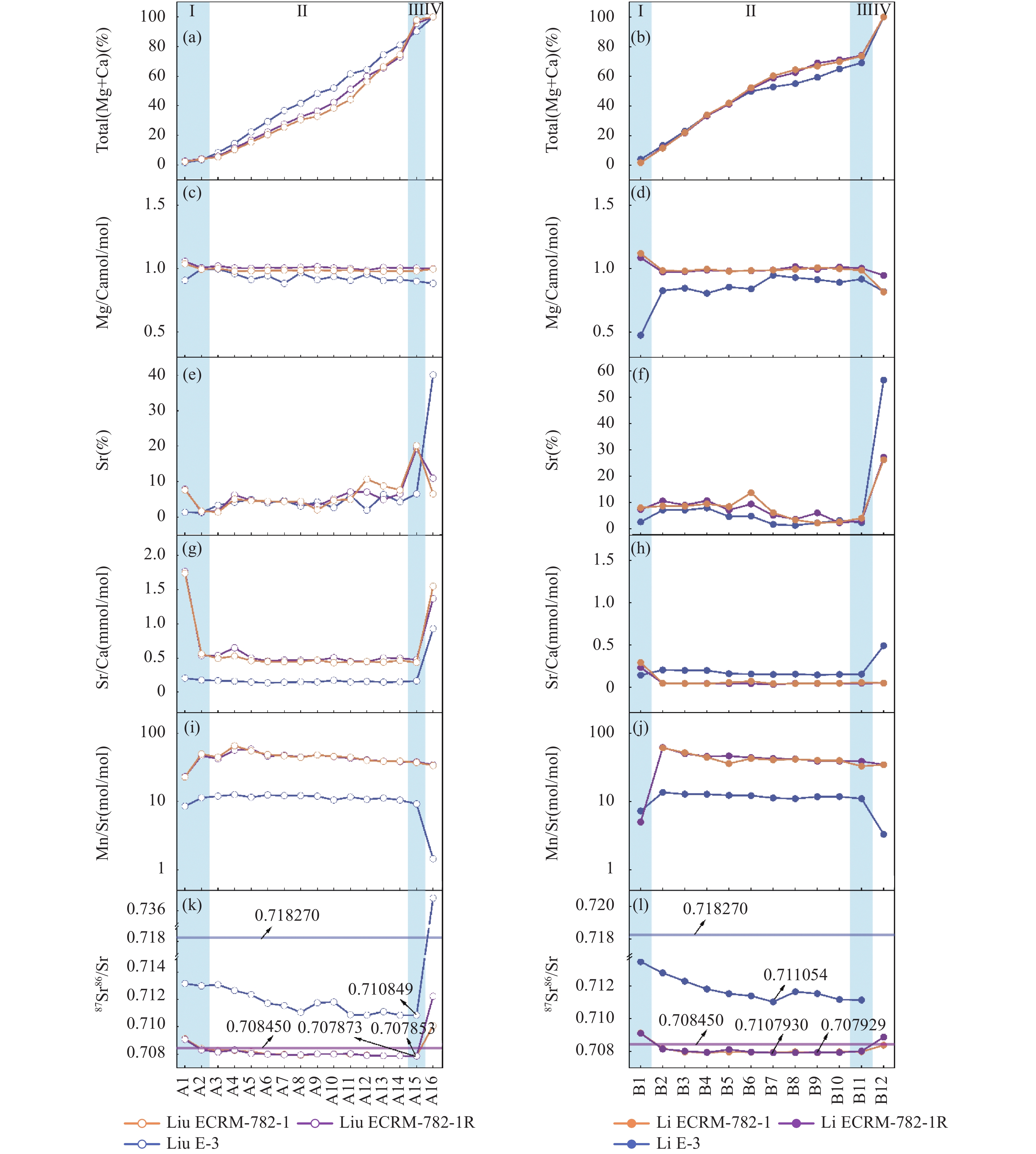
RESULTS (1) Factors affecting the Sr isotope composition of the primary carbonate fraction. The detection of Sr isotope composition of primary carbonate fraction is affected by the soluble and exchangeable Sr, resulting in higher 87Sr/86Sr values. Thus, the pre-leaching step is essential for the multiple-step leaching method. It is shown that an excess of 5% acetic acid can cause leaching of non-carbonate fraction of limestone and affect the Sr isotope composition of the primary carbonate fraction. (2) Comparison and selection of multiple-step extraction methods. (a) It is recommended to use the method proposed by Li, et al[29]for limestone samples. It was found that the carbonate of GBW03105a and C-3 dissolved in the target steps (A10-A11) proposed by Liu, et al[13] accounted for 46.51% and 39.49% of the total carbonate fraction. A large number of target carbonate was dissolved rapidly in these two steps without considerable differentiation. In the method proposed by Li, et al[29], GBW03105a and C-3 target steps (B7-B9) dissolved carbonate accounted for 26.41% and 30.31% of the total carbonate fraction. The target steps are close to the step of non-carbonate fractions dissolved in strong acid. For natural samples with complex mineral compositions, the method proposed by Li, et al[29] may have leached non-carbonate fractions, which resulted in a higher 87Sr/86Sr value for testing. Therefore, the more conservative method proposed by Li, et al[29] is recommended for leaching unknown limestone samples, and B7-B9 are the target steps for leaching representative primary carbonate fraction. (b) The method proposed by Liu, et al[13] is recommended for dolostone samples. Liu, et al[13] selected acetic acid with different concentration ranging from 0.25%-10% for leaching. The carbonate fraction is almost completely dissolved, and the lowest Sr isotope value measured is lower than the method proposed by Li, et al[29]. Hence, to choose the concentration of acetic acid from low to high for leaching is helpful to separate the target fractions of dolostone samples. The same concentration of acetic acid is chosen by the method of Li, et al[29], and the dissolution rate of samples with different purity began to slow down at A9, leaving about 30% of the carbonate fraction not leached. Thus, it is difficult to judge whether the target carbonate fraction using this method has been leached completely. For the multiple-step leaching of unknown dolostone samples, the method proposed by Liu, et al[13] is recommended here, and A14-A15 are selected as the target steps for leaching representative primary carbonate fraction. (3) Optimization of a multiple step leaching method. The insoluble powder in the leaching solution will be digested by the subsequent addition of nitric acid, which can affect the Sr isotope value of the target fraction. This study has found that the Al/Ca ratio in the acetic acid leaching part is lower than that in Li, et al[13] (Fig.3b), indicating that the filter can reduce pollution caused by the dissolution of non-target fractions. In addition, the pre-leaching steps can also be optimized. The experimental data showed that 5mL of 1mol/L ammonium acetate could be selected for pre-leaching to simplify the experimental procedure and shorten the processing time.
CONCLUSIONS Based on prior research, a focused multiple-step leaching method for carbonate rocks is proposed. Limestone samples (purity≥85%) are suitable for 9-step leaching with 1% acetic acid, and the target steps are L7-L9; dolomite samples (purity≥65%) are suitable for the 14-step leaching method with 0.25%-10% acetic acid, and the target steps are D13-D14. The Sr isotope value of the primary carbonate fraction of the European Committee for Steel Standardization (ECISS) dolostone reference material ECRM-782-1 has been reported for the first time, which is 0.707868±0.000034 (n=12, 2SD). In the future, the experimental methods will be further improved to encompass samples from diverse sources and varying purity, thereby ensuring the reliability and universality of the experimental approach.
-
Key words:
- carbonate rocks /
- Sr isotope /
- multiple-step leaching method /
- primary carbonate fraction /
- MC-ICP-MS
-

-
表 1 多步浸提实验步骤
Table 1. Procedures of the multiple-step leaching experiment.
步骤名称 步骤序号 各步所需时间 Liu等[13]提出方法 步骤序号 各步所需时间 Li等[29]提出方法 Ⅰ—乙酸铵预浸 A1、A2 30min 5mL 1mol/L乙酸铵+
0.02mL 8%过氧化氢B1 24h 10mL 1mol/L乙酸铵 Ⅱ—乙酸浸提 A3~A9 30min 5mL 0.25%乙酸 B2~B10 20min 3mL 1% 乙酸(灰岩)
4mL 2.5% 乙酸(白云岩)A10~A12 6mL 1%乙酸 A13~A14 3mL 5%乙酸 Ⅲ—过量乙酸浸提 A15 30min 6mL 10%乙酸 B11 20min 9mL 1% 乙酸(灰岩)
9mL 2.5% 乙酸(白云岩)Ⅳ—强酸溶解 A16 15h 1mL反王水+0.5mL氢氟酸 B12 15h 1mL反王水+0.5mL氢氟酸 表 2 采用Liu等[13]的方法各步浸提液元素比值及87Sr/86Sr比值
Table 2. Element and 87Sr/86Sr values in each step of the leaching procedure in Liu et al[13].
浸提
步骤样品GBW3105a 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)A1 0.709120 3 4 0.02 0.36 1.07 0.02 A2 0.709044 6 3 0.01 0.23 0.80 0.26 A3 0.709009 13 6 0.01 0.22 0.01 0.14 A4 0.709002 20 6 0.01 0.19 0.11 0.46 A5 0.708993 26 6 0.01 0.21 0.07 0.13 A6 0.708985 33 6 0.01 0.23 0.03 0.27 A7 0.708974 39 6 0.01 0.23 0.09 0.21 A8 0.708941 46 6 0.01 0.21 0.07 0.43 A9 0.708949 52 6 0.01 0.22 0.07 0.24 A10 0.708889 77 25 0.01 0.23 0.04 0.37 A11 0.708920 99 22 0.03 0.25 0.17 0.44 A12 - 99 0 0.73 0.09 23.46 8.24 A13 - 100 0 0.91 0.05 29.57 15.72 A14 - 100 0 0.88 0.28 68.51 3.86 A15 - 100 0 0.39 1.04 0.00 3.06 A16 - 100 2 0.62 6.22 7.65 0.73 浸提
步骤样品C-3 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)A1 0.709862 3 4 0.02 0.93 0.29 0.34 A2 0.709773 7 3 0.02 0.81 0.43 0.40 A3 0.709721 14 7 0.02 0.90 0.01 0.17 A4 0.709712 21 7 0.02 0.84 0.01 0.48 A5 0.709702 29 7 0.02 0.83 0.01 0.48 A6 0.709707 37 8 0.02 0.86 0.01 0.47 A7 0.709696 45 8 0.02 0.88 0.02 0.46 A8 0.709709 52 7 0.02 0.88 0.02 0.25 A9 0.709680 59 7 0.02 0.90 0.03 0.25 A10 0.709655 75 16 0.04 0.86 0.00 0.55 A11 0.709655 98 23 0.02 0.86 0.05 0.50 A12 0.709807 99 1 0.12 0.94 24.14 0.67 A13 0.710330 100 1 0.39 0.83 49.37 1.40 A14 - 100 0 0.79 0.87 191.98 2.71 A15 - 100 0 0.67 2.58 1952.20 1.05 A16 - 100 0 8.06 1.93 32.74 3.08 浸提
步骤样品ECRM-782-1 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)A1 0.709127 3 8 1.06 1.77 3.74 23.15 A2 0.708408 4 2 1.01 0.54 4.04 46.89 A3 0.708270 6 2 1.02 0.54 5.73 42.24 A4 0.708276 11 6 1.00 0.65 1.84 56.58 A5 0.708185 17 5 1.00 0.50 0.70 58.77 A6 0.708010 22 4 1.01 0.46 0.72 46.22 A7 0.707987 27 4 1.00 0.47 1.51 47.75 A8 0.707913 32 4 1.01 0.47 1.70 44.70 A9 0.708009 36 3 1.02 0.47 7.40 48.15 A10 0.708018 42 5 1.00 0.50 16.67 45.40 A11 0.708000 51 7 1.00 0.45 8.34 43.13 A12 0.707935 60 7 0.99 0.45 7.70 40.91 A13 0.707875 65 5 1.01 0.50 13.95 38.91 A14 0.707880 73 7 1.00 0.50 8.80 38.36 A15 0.707873 96 19 1.00 0.48 6.45 38.10 A16 0.710082 100 11 1.00 1.37 469.94 34.58 (续表 2) 浸提
步骤样品ECRM-782-1R 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)A1 0.709080 2 8 1.04 1.74 3.30 22.91 A2 0.708318 4 2 0.99 0.56 4.47 49.75 A3 0.708155 5 1 1.00 0.49 5.16 44.60 A4 0.708311 10 5 0.98 0.53 0.85 65.37 A5 0.708051 15 5 0.98 0.46 1.16 55.30 A6 0.707996 20 5 0.98 0.45 0.73 48.69 A7 0.707956 25 4 0.99 0.45 1.02 46.70 A8 0.707944 30 4 0.99 0.45 1.23 44.30 A9 0.708024 33 2 0.99 0.47 18.25 48.09 A10 0.708005 38 5 0.98 0.43 11.40 45.70 A11 0.708036 44 5 0.99 0.44 7.58 44.59 A12 0.707903 56 11 0.98 0.45 5.39 40.16 A13 0.707875 66 9 0.98 0.44 8.74 38.81 A14 0.707873 75 8 0.98 0.46 7.34 39.00 A15 0.707853 98 20 0.98 0.44 5.50 37.25 A16 0.712228 100 7 1.00 1.55 1717.39 33.68 浸提
步骤样品E-3 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)A1 0.713153 2 1 0.91 0.20 0.00 8.55 A2 0.712982 3 1 1.00 0.18 0.00 11.37 A3 0.713075 8 3 1.00 0.17 0.42 12.00 A4 0.712645 14 4 0.96 0.16 0.17 12.64 A5 0.712345 22 5 0.91 0.15 0.06 11.55 A6 0.711715 29 4 0.94 0.14 0.13 12.48 A7 0.711543 36 5 0.88 0.15 0.05 12.23 A8 0.711056 41 3 0.97 0.15 0.49 12.17 A9 0.711750 48 4 0.91 0.15 0.10 11.98 A10 0.711815 51 3 0.94 0.17 1.21 10.53 A11 0.710880 61 6 0.91 0.15 0.20 11.68 A12 0.710850 64 2 0.95 0.16 0.47 10.75 A13 0.711104 74 6 0.91 0.15 0.25 11.27 A14 0.710849 81 4 0.91 0.15 0.32 10.50 A15 0.710849 90 7 0.90 0.16 0.33 9.22 A16 0.736939 100 40 0.88 0.93 411.08 1.45 表 3 采用Li等[29]方法各步浸提液元素比值及87Sr/86Sr比值
Table 3. Element and 87Sr/86Sr values in each step of the leaching procedure in Li et al[29].
浸提
步骤样品GBW3105a 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)B1 0.709095 4 7 0.02 0.43 0.17 0.01 B2 0.709012 12 8 0.01 0.21 0.01 0.26 B3 0.709025 22 8 0.01 0.20 0.02 0.40 B4 0.708964 31 8 0.01 0.20 0.01 0.61 B5 0.708934 40 9 0.01 0.21 0.01 0.38 B6 0.708926 49 9 0.01 0.21 0.01 0.38 B7 0.708877 58 8 0.01 0.22 0.05 0.34 B8 0.708878 67 9 0.01 0.22 0.06 0.34 B9 0.708877 76 9 0.01 0.22 0.04 0.30 B10 0.708881 85 9 0.01 0.23 0.05 0.34 B11 0.708912 99 15 0.04 0.24 0.72 0.45 B12 0.730411 100 2 0.92 0.41 364.51 3.20 (续表 3) 浸提
步骤样品C-3 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)B1 0.709875 5 5 0.02 0.96 0.13 0.12 B2 0.709711 15 10 0.02 0.87 0.00 0.53 B3 0.709731 25 10 0.02 0.82 0.00 0.49 B4 0.709734 36 11 0.02 0.87 0.00 0.48 B5 0.709686 47 11 0.02 0.87 0.00 0.48 B6 0.709682 58 11 0.02 0.86 0.00 0.49 B7 0.709666 69 10 0.02 0.83 0.00 0.48 B8 0.709675 79 11 0.02 0.87 0.00 0.49 B9 0.709670 88 9 0.02 0.86 0.07 0.44 B10 0.709677 95 7 0.04 0.83 0.53 0.53 B11 0.709831 99 4 0.08 0.84 6.55 0.61 B12 0.709652 100 0 2.30 0.35 12017.92 6.68 浸提
步骤样品ECRM-782-1 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)B1 0.709137 2 7 1.09 0.23 0.19 5.00 B2 0.708186 12 10 0.97 0.05 0.65 61.79 B3 0.707971 22 9 0.98 0.04 0.57 50.06 B4 0.707958 33 11 0.99 0.04 0.47 45.70 B5 0.707977 41 7 0.98 0.04 0.61 46.46 B6 0.707963 51 9 0.98 0.04 0.44 43.91 B7 0.707957 59 5 0.99 0.03 0.48 42.60 B8 0.707988 62 4 1.02 0.04 0.80 41.94 B9 0.707929 69 6 0.99 0.04 0.63 38.88 B10 0.707988 71 2 1.01 0.05 0.95 38.87 B11 0.707994 74 3 1.00 0.05 0.89 38.81 B12 0.708390 100 27 0.95 0.05 3.21 34.72 浸提
步骤样品ECRM-782-1R 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)B1 0.709120 2 8 1.12 0.29 0.01 0.00 B2 0.708159 11 9 0.98 0.05 0.61 60.99 B3 0.708030 22 9 0.98 0.04 0.54 51.85 B4 0.707947 34 9 1.00 0.04 0.43 44.25 B5 0.708126 42 8 0.98 0.05 0.82 35.92 B6 0.707974 52 14 0.99 0.07 0.69 42.64 B7 0.707930 60 6 0.99 0.04 0.53 40.70 B8 0.707934 64 3 0.99 0.04 0.70 41.49 B9 0.707945 67 2 1.01 0.05 0.80 40.09 B10 0.707945 70 2 1.00 0.04 0.65 39.85 B11 0.708029 74 4 0.99 0.05 0.90 32.85 B12 0.70888 100 26 0.82 0.05 5.28 34.60 浸提
步骤样品E-3 87Sr/ 86Sr 总Ca
(%)Sr
(%)Mg/Ca
(mmol/mol)Sr/Ca
(mmol/mol)Al/Ca
(mmol/mol)Mn/Sr
(mol/mol)B1 0.713510 4 2 0.47 0.14 0.06 7.26 B2 0.712825 13 7 0.83 0.20 0.94 13.58 B3 0.712315 23 7 0.85 0.20 0.47 12.81 B4 0.711839 33 8 0.81 0.20 0.32 12.76 B5 0.711553 41 5 0.85 0.16 0.37 12.31 B6 0.711417 50 5 0.84 0.15 0.35 12.18 B7 0.711054 53 2 0.95 0.15 0.60 11.27 B8 0.711674 55 1 0.93 0.15 0.67 10.99 B9 0.711559 59 2 0.91 0.14 0.41 11.75 B10 0.711196 65 3 0.89 0.15 0.42 11.79 B11 0.711166 69 2 0.92 0.15 0.70 11.00 B12 - 100 56 0.82 0.49 128.22 3.30 注:“-”表示低于检测限。 表 4 样品基本信息
Table 4. Basic information of samples.
样品编号 岩石种类 全岩Sr含量
(μg/g)全岩87Sr/86Sr比值 碳酸盐纯度
(%)GBW03105a 灰岩 216.64 0.709161±0.000017 96 C-3 灰岩 675.64 0.710180 85 ECRM-782-1 白云岩 24.68 0.708452±0.000009 99 E-3 白云岩 65.54 0.718270 65 表 5 优化后的多步浸提法实验步骤
Table 5. Optimized experimental procedures for multiple-step leaching method.
步骤序号 各步所需时间
(min)白云岩浸提流程 D1 30 5mL 1mol/L乙酸铵+0.02mL 8%过氧化氢 D2~D8 30 5mL 0.25%乙酸 D9~D11 30 6mL 1%乙酸 D12~D13 30 3mL 5%乙酸 D14 30 6mL 10%乙酸 步骤序号 各步所需时间
(min)灰岩浸提流程 L1 30 5mL 1mol/L乙酸铵+0.02mL 8%过氧化氢 L2~L9 20 3mL 1%乙酸 表 6 采用优化后浸提法标准物质的Sr同位素结果
Table 6. The Sr isotope results of standard material using optimized leaching method.
标准物质编号 87Sr/86Sr比值 Sr同位素偏差 GBW03105a 0.708930±0.000015(n=12,2SD) 0.000053 ECRM-782-1 0.707868±0.000034(n=12,2SD) 0.000015 -
[1] Mcarthur J M, Howarth R J, Shields G A. Strontium isotope stratigraphy[J]. The Geologic Time Scale, 2012: 127-144.
[2] Kuznetsov A B, Semikhatov M A, Gorokhov I M. The Sr isotope composition of the world ocean, marginal and inland seas: Implications for the Sr isotope stratigraphy[J]. Stratigraphy and Geological Correlation, 2012, 20(6): 501−515. doi: 10.1134/S0869593812060044
[3] Jones C E, Jenkyns H C. Seawater strontium isotopes, oceanic anoxic events, and seafloor hydrothermal activity in the Jurassic and Cretaceous[J]. American Journal of Science, 2001, 301(2): 112−149. doi: 10.2475/ajs.301.2.112
[4] Palmer M R, Edmond J M. The strontium isotope budget of the modern ocean[J]. Earth and Planetary Science Letters, 1989, 92(1): 11−26. doi: 10.1016/0012-821X(89)90017-4
[5] Allègre C J, Louvat P, Gaillardet J, et al. The fundamental role of island arc weathering in the oceanic Sr isotope budget[J]. Earth and Planetary Science Letters, 2010, 292(1-2): 51−56. doi: 10.1016/j.jpgl.2010.01.019
[6] Schildgen T F, Cosentino D, Frijia G, et al. Sea level and climate forcing of the Sr isotope composition of late Miocene Mediterranean marine basins[J]. Geochemistry, Geophysics, Geosystems, 2014, 15(7): 2964−2983.
[7] Peucker-Ehrenbrink B, Fiske G J. A continental perspective of the seawater 87Sr/86Sr record: A review[J]. Chemical Geology, 2019, 510: 140−165. doi: 10.1016/j.chemgeo.2019.01.017
[8] Zaky A H, Brand U, Buhl D, et al. Strontium isotope geochemistry of modern and ancient archives: Tracer of secular change in ocean chemistry[J]. Canadian Journal of Earth Sciences, 2019, 56(3): 245−264. doi: 10.1139/cjes-2018-0085
[9] Mcarthur J M, Howarth R J, Shields G A, et al. Strontium isotope stratigraphy[M]. Geologic Time Scale, 2020: 211-238.
[10] Derry L A, Kaufman A J, Jacobsen S B. Sedimentary cycling and environmental change in the late Proterozoic: Evidence from stable and radiogenic isotopes[J]. Geochimica et Cosmochimica Acta, 1992, 56(3): 1317−1329. doi: 10.1016/0016-7037(92)90064-P
[11] Shields G, Veizer J. Precambrian marine carbonate isotope database: Version 1.1[J]. Geochemistry, Geophysics, Geosystems, 2002, 3(6): 1−12.
[12] 张志军, 尹观, 张其春, 等. 碳酸盐岩锶同位素比值测定中的残渣分析[J]. 岩矿测试, 2003, 22(2): 151−153. doi: 10.3969/j.issn.0254-5357.2003.02.015
Zhang Z J, Yin G, Zhang Q C, et al. The residue analysis in determination of Sr isotopes ratio of carbonate[J]. Rock and Mineral Analysis, 2003, 22(2): 151−153. doi: 10.3969/j.issn.0254-5357.2003.02.015
[13] Liu C, Wang Z R, Raub T D. Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia[J]. Chemical Geology, 2013, 351: 95−104. doi: 10.1016/j.chemgeo.2013.05.012
[14] Edwards C T, Saltzman M R, Leslie S A, et al. Strontium isotope (87Sr/86Sr) stratigraphy of Ordovician bulk carbonate: Implications for preservation of primary seawater values[J]. Geological Society of America Bulletin, 2015, 127(9): 1275−1289.
[15] Fairchild I J, Spencer A M, Ali D O, et al. Tonian—Cryogenian boundary sections of Argyll, Scotland[J]. Precambrian Research, 2018, 319: 37−64. doi: 10.1016/j.precamres.2017.09.020
[16] Hood A, Wallace M W. Neoproterozoic marine carbonates and their paleoceanographic significance[J]. Global and Planetary Change, 2017, 160: 28−45.
[17] Li D, Shields-Zhou G A, Ling H F, et al. Dissolution methods for strontium isotope stratigraphy: Guidelines for the use of bulk carbonate and phosphorite rocks[J]. Chemical Geology, 2011, 290(3-4): 133−144. doi: 10.1016/j.chemgeo.2011.09.004
[18] Zhang K, Zhu X K, Yan B. A refined dissolution method for rare earth element studies of bulk carbonate rocks[J]. Chemical Geology, 2015, 412: 82−91. doi: 10.1016/j.chemgeo.2015.07.027
[19] Azmy K, Kaufman A J, Misi A, et al. Isotope stratigraphy of the Lapa Formation, São Francisco Basin, Brazil: Implications for late Neoproterozoic glacial events in South America[J]. Precambrian Research, 2006, 149(3-4): 231−248. doi: 10.1016/j.precamres.2006.07.001
[20] Nogueira A, Riccomini C, Sial A N, et al. Carbon and strontium isotope fluctuations and paleoceanographic changes in the late Neoproterozoic Araras carbonate platform, Southern Amazon Craton, Brazil[J]. Chemical Geology, 2007, 237(1-2): 168−190. doi: 10.1016/j.chemgeo.2006.06.016
[21] Galindo C, Casquet C, Rapela C, et al. Sr, C and O isotope geochemistry and stratigraphy of Precambrian and lower Paleozoic carbonate sequences from the Western Sierras Pampeanas of Argentina: Tectonic implications[J]. Precambrian Research, 2004, 131(1): 55−71.
[22] Miller N, Johnson P R, Stern R J. Marine versus non-marine environments for the Jibalah Group, NW Arabian shield: A sediment logic and geochemical survey and report of possible metazoa in the Dhaiqa Formation[J]. Arabin Journal for Science and Engineering, 2008, 33(1): 55−77.
[23] Sawaki Y, Kawai T, Shibuya T, et al. 87Sr/86Sr chemostratigraphy of Neoproterozoic Dalradian carbonates below the Port Askaig Glaciogenic Formation, Scotland[J]. Precambrian Research, 2010, 179(1-4): 150−164. doi: 10.1016/j.precamres.2010.02.021
[24] Zhang Y G, Yang T, Hohl S V, et al. Seawater carbon and strontium isotope variations through the late Ediacaran to late Cambrian in the Tarim Basin[J]. Precambrian Research, 2020, 345: 105769. doi: 10.1016/j.precamres.2020.105769
[25] Bailey T R, Mcarthur J M, Prince H, et al. Dissolution methods for strontium isotope stratigraphy: Whole rock analysis[J]. Chemical Geology, 2000, 167(3): 313−319.
[26] Bellefroid E J, Planavsky N J, Miller N R, et al. Case studies on the utility of sequential carbonate leaching for radiogenic strontium isotope analysis[J]. Chemical Geology, 2018, 497: 88−99. doi: 10.1016/j.chemgeo.2018.08.025
[27] Cui H, Kaufman A J, Xiao S, et al. Redox architecture of an Ediacaran ocean margin: Integrated chemostratigraphic (δ13C-δ34S-87Sr/86Sr-Ce/Ce*) correlation of the Doushantuo Formation, South China[J]. Chemical Geology, 2015, 405: 48−62. doi: 10.1016/j.chemgeo.2015.04.009
[28] Kochnev B B, Pokrovsky B G, Kuznetsov A B, et al. C and Sr isotope chemostratigraphy of Vendian—lower Cambrian carbonate sequences in the Central Siberian Platform[J]. Russian Geology and Geophysics, 2018, 59(6): 585−605. doi: 10.1016/j.rgg.2018.05.001
[29] Li Y L, Li C F, Guo J H. Re-evaluation and optimisation of dissolution methods for strontium isotope stratigraphy based on chemical leaching of carbonate certificated reference materials[J]. Microchemical Journal, 2020, 154: 104607. doi: 10.1016/j.microc.2020.104607
[30] Brand U, Jiang G, Azmy K, et al. Diagenetic evaluation of a Pennsylvanian carbonate succession (Bird Spring Formation, Arrow Canyon, Nevada, U. S. A. )—1: Brachiopod and whole rock comparison[J]. Chemical Geology, 2012, 308-309: 26−39. doi: 10.1016/j.chemgeo.2012.03.017
[31] 王意茹, 武晓郯, 何静, 等. 碳酸盐矿物中稀土元素分馏特征及其获取方法研究进展[J]. 岩矿测试, 2022, 41(6): 935−946. doi: 10.15898/j.cnki.11-2131/td.202204180081
Wang Y R, Wu X T, He J, et al. A review of research progress on fractionation characteristics and acquisition methods of rare earth elements in carbonate minerals[J]. Rock and Mineral Analysis, 2022, 41(6): 935−946. doi: 10.15898/j.cnki.11-2131/td.202204180081
[32] Liu C, Wang Z, Raub T D, et al. Neoproterozoic cap-dolostone deposition in stratified glacial meltwater plume[J]. Earth and Planetary Science Letters, 2014, 404: 22−32. doi: 10.1016/j.jpgl.2014.06.039
[33] Wen B, Evans D A D, Li Y X, et al. Newly discovered Neoproterozoic diamictite and cap carbonate (DCC) couplet in Tarim Craton, NW China: Stratigraphy, geochemistry, and paleoenvironment[J]. Precambrian Research, 2015, 271: 178−294.
[34] Romero J A S, Lafon J M, Nogueira A C R, et al. Sr isotope geochemistry and Pb-Pb geochronology of the Neoproterozoic cap carbonates, Tangará da Serra, Brazil[J]. International Geology Review, 2013, 55(2): 1−19.
[35] Kong J J, Niu Y L, Sun P, et al. The origin and geodynamic significance of the Mesozoic dykes in eastern continental China[J]. Lithos, 2019, 332-333: 328−339. doi: 10.1016/j.lithos.2019.02.024
[36] Chen S, Wang X H, Niu Y L, et al. Simple and cost-effective methods for precise analysis of trace element abundances in geological materials with ICP-MS[J]. Science Bulletin, 2017, 62(4): 277−289. doi: 10.1016/j.scib.2017.01.004
[37] Sun P, Niu Y L, Guo P Y, et al. Multiple mantle metasomatism beneath the Leizhou Peninsula, South China: Evidence from elemental and Sr-Nd-Pb-Hf isotope geochemistry of the late Cenozoic volcanic rocks[J]. International Geology Review, 2019, 61(14): 1768−1785. doi: 10.1080/00206814.2018.1548307
[38] Lv Y W, Liu S A, Wu H C, et al. Zn-Sr isotope records of the Ediacaran Doushantuo Formation in South China: Diagenesis assessment and implications[J]. Geochimica et Cosmochimica Acta, 2018, 239: 330−345. doi: 10.1016/j.gca.2018.08.003
[39] Frimmel H E. On the reliability of stable carbon isotopes for Neoproterozoic chemostratigraphic correlation[J]. Precambrian Research, 2010, 182(4): 239−253. doi: 10.1016/j.precamres.2010.01.003
[40] 赵彦彦, 郑永飞. 碳酸盐沉积物的成岩作用[J]. 岩石学报, 2011, 27(2): 501−519.
Zhao Y Y, Zheng Y F. Diagenesis of carbonate sediments[J]. Acta Petrologica Sinica, 2011, 27(2): 501−519.
-




 下载:
下载: