Selective Separation of Scandium in Acidic Water Using Carboxyl Functionalized Covalent Phosphonitrile Polymers
-
摘要:
发展高效选择性分离技术实现酸性介质中稀土元素钪(Sc)的高效提取,有利于满足工业生产过程对Sc日益增长的需求。吸附法可以有效地简化Sc的提取流程,降低生产成本。然而,现有吸附材料如硅基材料、生物质材料、金属有机骨架材料等在分离酸性介质中的Sc时仍存在效率低、选择性差等缺点,从而限制了其实际应用。因此,针对处理含Sc尾矿等原料时往往需要进行酸处理的实际情况,制备具备更强结合能力的吸附剂,实现酸性介质中Sc(III)的高效选择性分离至关重要。本文分别以间苯三酚和2,4,6-三羟基苯甲酸为构筑单元,与六氯三聚膦腈共价交联制备了共价膦腈聚合物(covalent phosphonitrile frameworks,CPF-T)和羧基功能化CPF材料(CPF-T-COOH)。采用扫描电镜、红外光谱、热重、氮气吸附-解吸等分析技术对材料的结构进行表征,并探索了它们作为Sc(III)吸附剂的应用潜力。吸附实验结果表明,在溶液pH=2时,CPF-T和CPF—COOH对Sc(III)的吸附平衡时间分别为120min和30min,最大吸附容量分别为22.48mg/g和64.63mg/g,在混合离子溶液中的分配系数分别为5.1×102mL/g和4.0×103mL/g。值得注意的是,在酸性介质(pH=1~3)中,CPF-T-COOH对Sc(III)的吸附率仍维持在95%以上,明显优于CPF-T(低于60%)以及大多数已报道的吸附材料。采用X射线光电子能谱对作用机理进一步分析,结果显示除了CPF-T骨架中N/O原子与Sc(III)的配位作用外,骨架—COOH上的O原子与-OH基团分别提供了额外的配位作用与离子交换作用,有效地增强了CPF—COOH对Sc(III)的吸附亲和力。该研究表明,利用多种功能基团的协同作用是提高吸附剂吸附性能的有效策略之一,所制备的CPF-T-COOH材料性能优异,展现了其作为Sc(III)吸附材料的良好应用前景。
Abstract:BACKGROUND Scandium (Sc) is widely used due to its excellent properties such as high melting point, high boiling point, low density, and good stability. However, as a typical dispersed element, Sc usually exists as an associated mineral. Sc needs to be recovered from the production of ore residues and tailings by-products of other metals, the production of main products from Sc containing internal (external) deposits, or from industrial wastewater and waste residue. Among numerous technologies for separating Sc, the adsorption method exhibits promising prospects due to its advantages of simple operation and high recovery. Currently, several materials including silicon-based (such as SAB-15), biomaterials, and metal organic framework, have been used for the separation of Sc(III). Although these adsorbents exhibit good adsorption ability for Sc(III), the inherent structural drawbacks such as poor chemical stability and with only one type of functional group result in the poor selectivity and inferior adsorption capacity, thereby greatly hindering their practical applications. Considering that acid treatment is often required when processing raw materials such as Sc containing tailings, it is crucial to prepare adsorbents that can efficiently and selectively recover Sc(III) in acidic media. Covalent organic polymers are porous organic polymer materials connected by covalent bonds. Due to their adjustable chemical structure, tunable pore size, and easy functionalization, they can be custom-made according to the characteristics of target ions. As one type of alternative adsorbents, they exhibit promising prospects. The pore size matching effect is conducive to achieving the efficient adsorption of target ions in acidic systems. However, because the pore size of the vast majority of covalent organic polymers currently synthesized is much larger than those of metal ions, this method is generally applicable to larger sized ions such as hydrated uranium ions. The utilization of ion imprinting technology or the ingenious selection of monomers with size matching cavities with the target ion to prepare covalent organic polymers are effective for address these issues. However, the complex preparation process makes it difficult to scale up. For covalent organic polymers, modification with special functional groups is another effective strategy to improve their adsorption performance. To tackle the issue of limited binding ability caused by the single functional group, research has shown that the introduction of various functional groups into the porous skeleton structure can effectively enhance the selective binding ability to target ions by utilizing the synergistic effect between different groups.
OBJECTIVES To improve the adsorption performance of porous organic polymers for Sc(III) in acidic media by utilizing the synergistic effect of multiple functional groups.
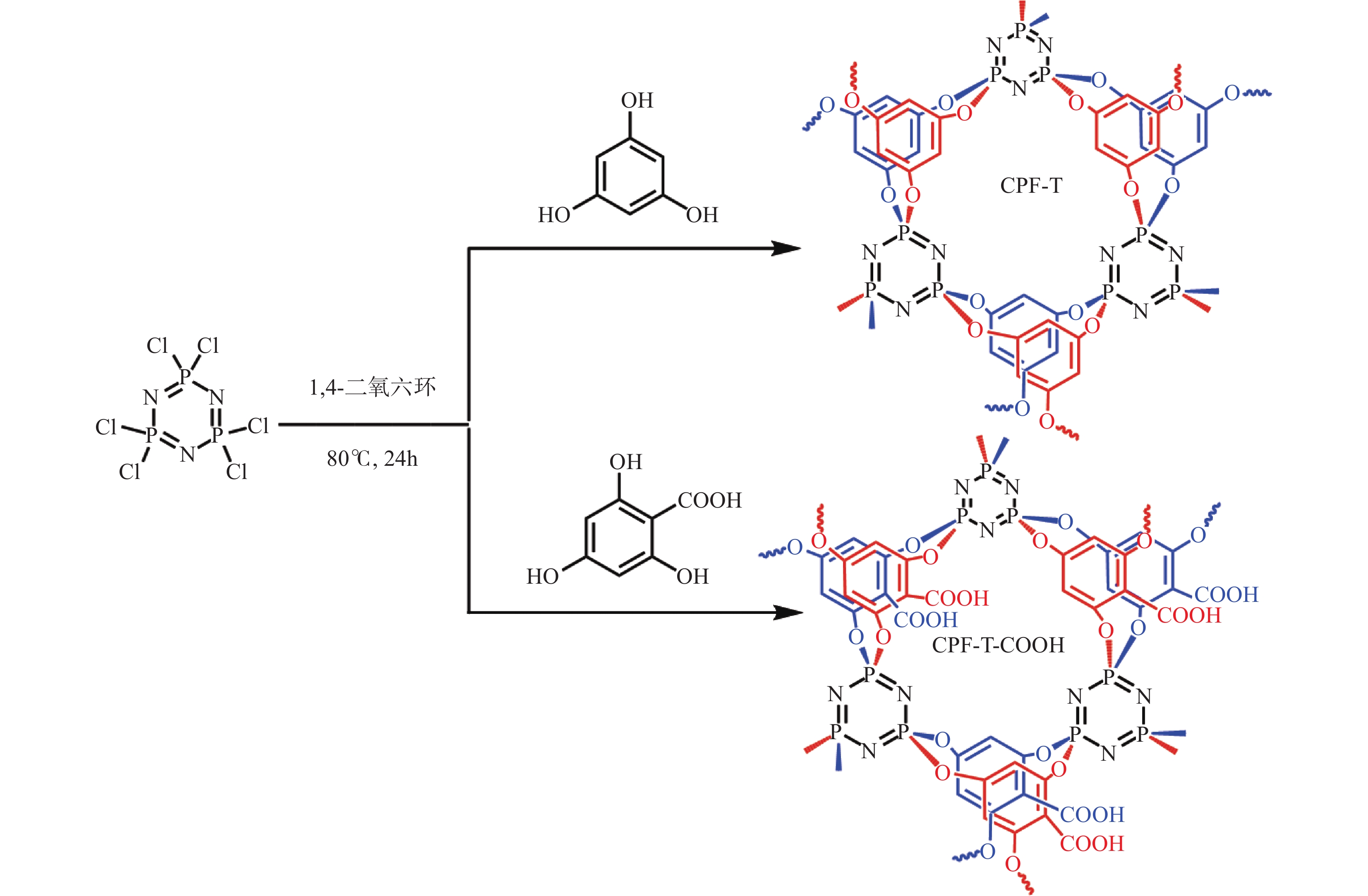
METHODS Phloroglucinol (1mmol) and hexachlorocyclotriphosphazene (0.5mmol) were dissolved in 1mL of 1,4-dioxane, followed by the addition of 0.84mL of triethylamine. The mixture was transferred to a hydrothermal reactor (8mL) and reacted at 80℃ for 24h. The product was washed several times with water, ethanol and acetone, respectively. Finally, CPF-T was prepared by vacuum drying at 50℃ for 12h. CPF-T-COOH was prepared according to the same procedure by changing phloroglucinol with 2,4,6-trihydroxybenzoic acid. The infrared spectra of the CPF-T and CPF-T-COOH were collected by Thermo Scientific Nicolet iS20 Fourier transform infrared spectrometer (FT-IR) (Thermo, USA). The microstructure of the polymers was studied using SU8010 scanning electron microscope (SEM) (Hitachi, Japan). The thermogravimetric analysis (TGA) curve of the polymers was collected by the DTA7200 TGA instrument (Hitachi, Japan) under N2 conditions (N2 flow rate: 20mL/min; heating rate: 10K/min). The elemental information of the materials was analyzed using Thermo Scientific K-Alpha X-ray photoelectron spectrometer (XPS) (Thermo, USA). The N2 adsorption desorption isotherm was measured at 77K using the Micromeritics APSP2460 4-station fully automatic specific surface area analyzer (Micromeritics, USA). The sample was vacuum degassed at 120℃ for 12h before measurement. Adsorption experiments were conducted at room temperature using a constant temperature oscillator (150r/min). The adsorbent dosage was 1g/L, and the experimental data was obtained as the average of three parallel experiments. After adsorption, the supernatant was collected by filtration with 0.45μm microporous membrane. Subsequently, it was tested by EXPEC 6000 ICP-OES (Hangzhou Puyu Technology Development Co., Ltd.), and the linear correlation coefficient (R2) of the standard curve equation was >0.999.
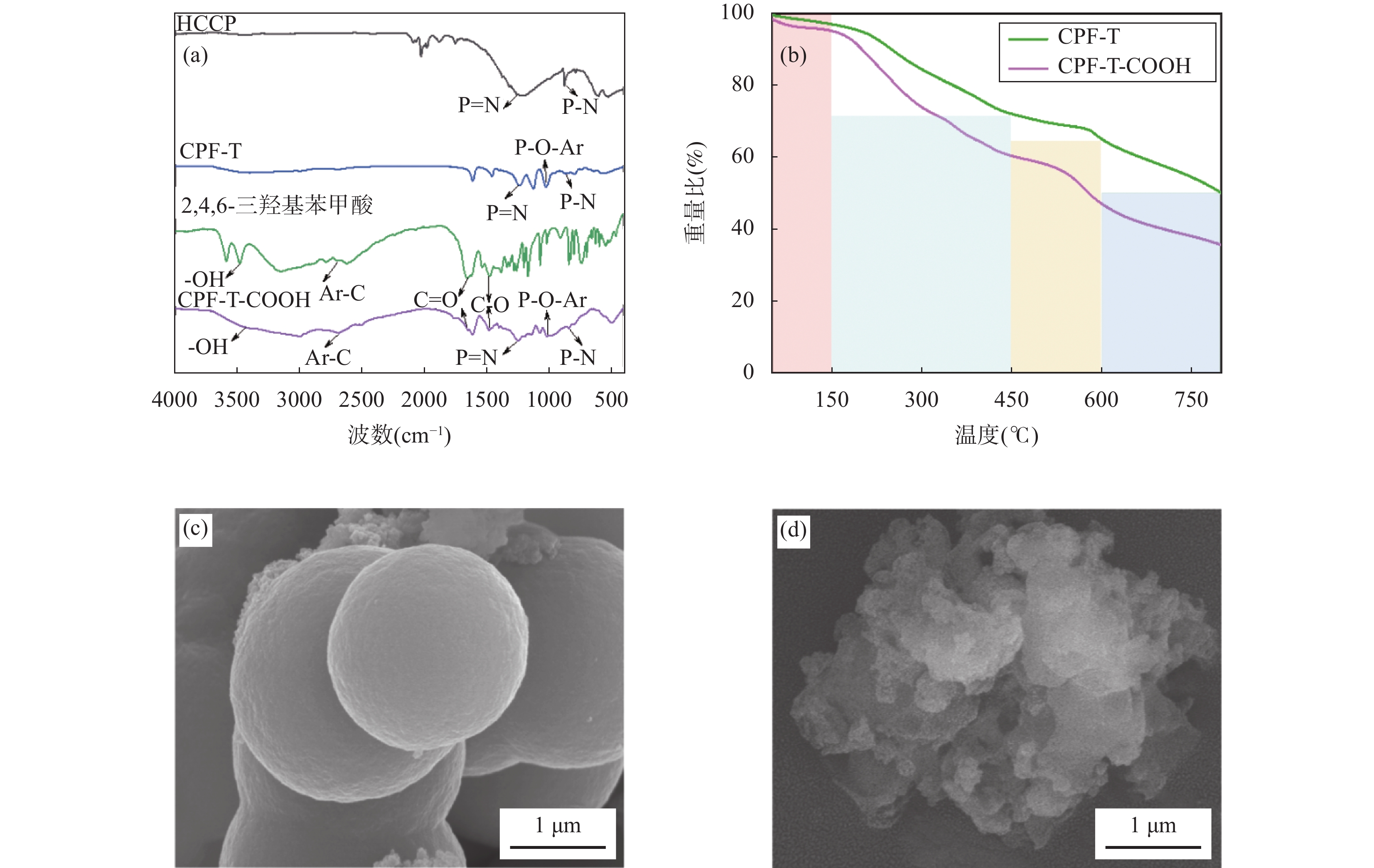
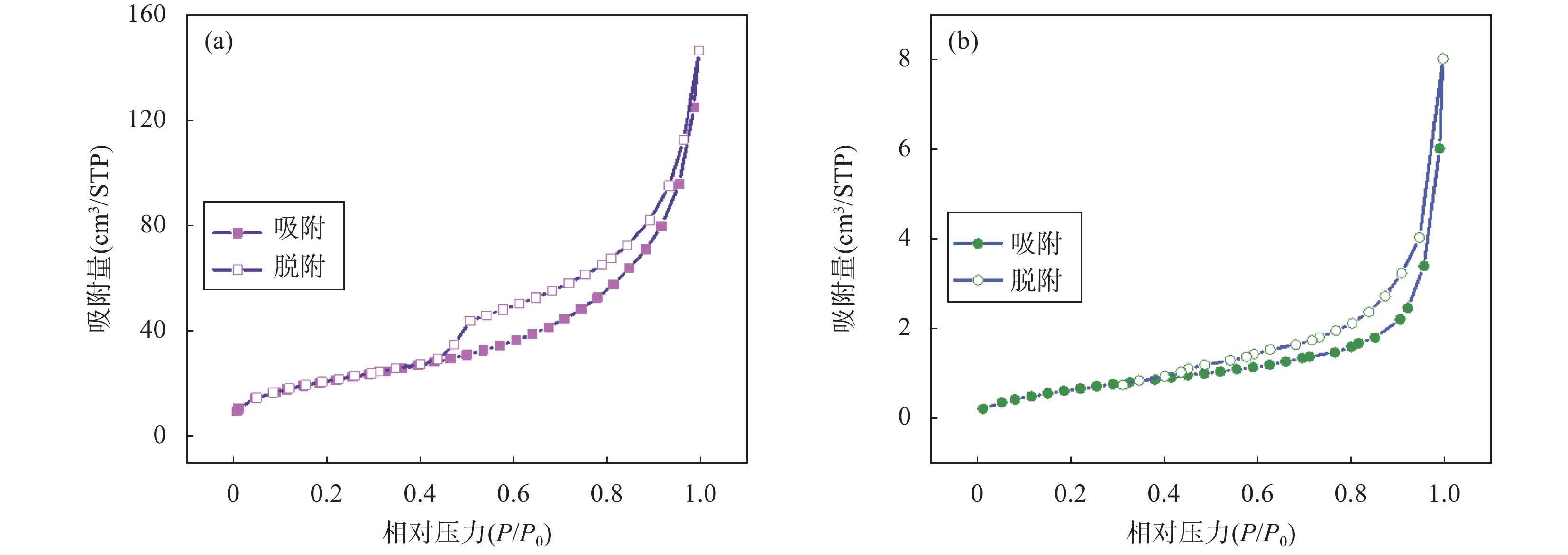
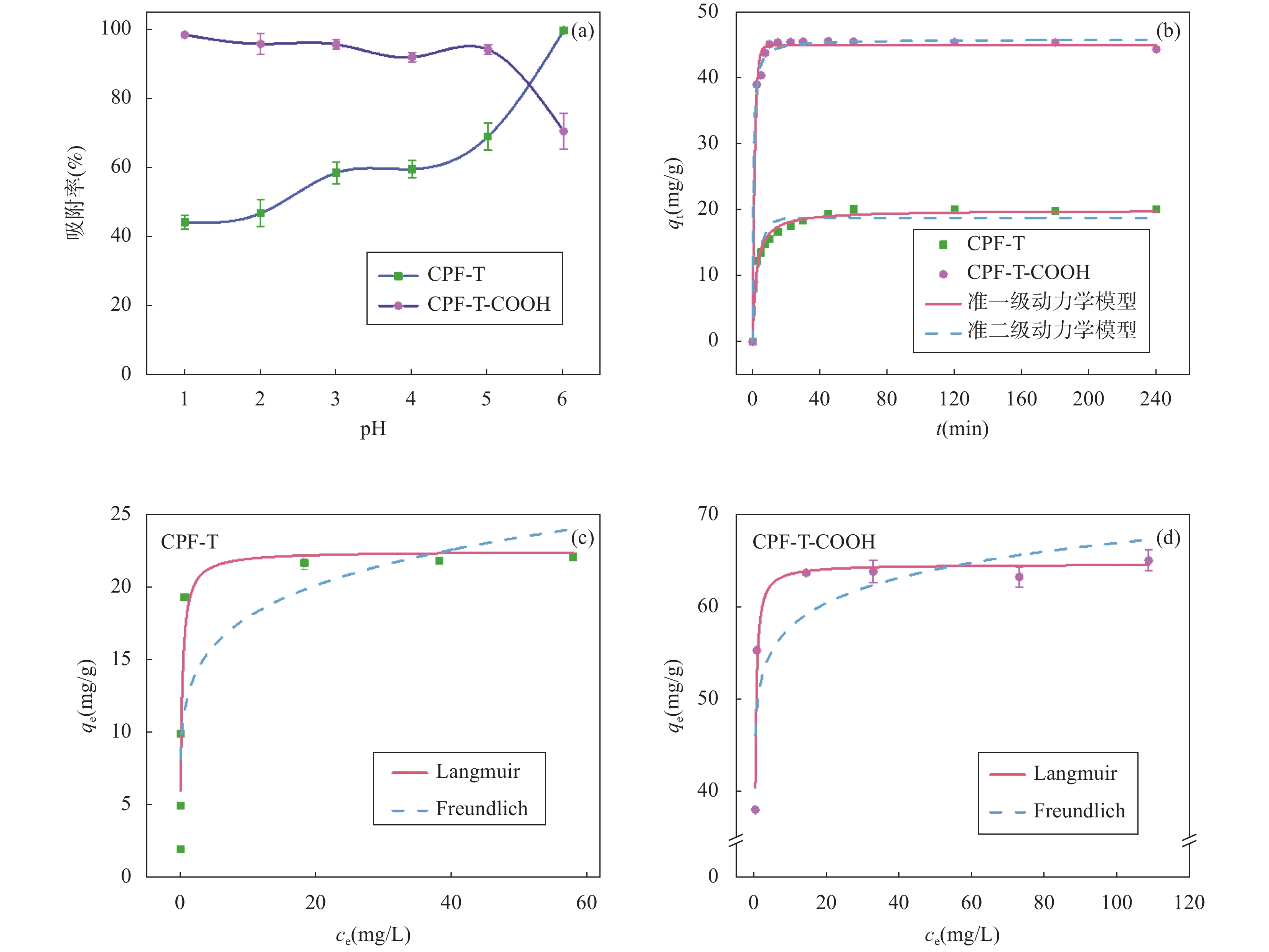
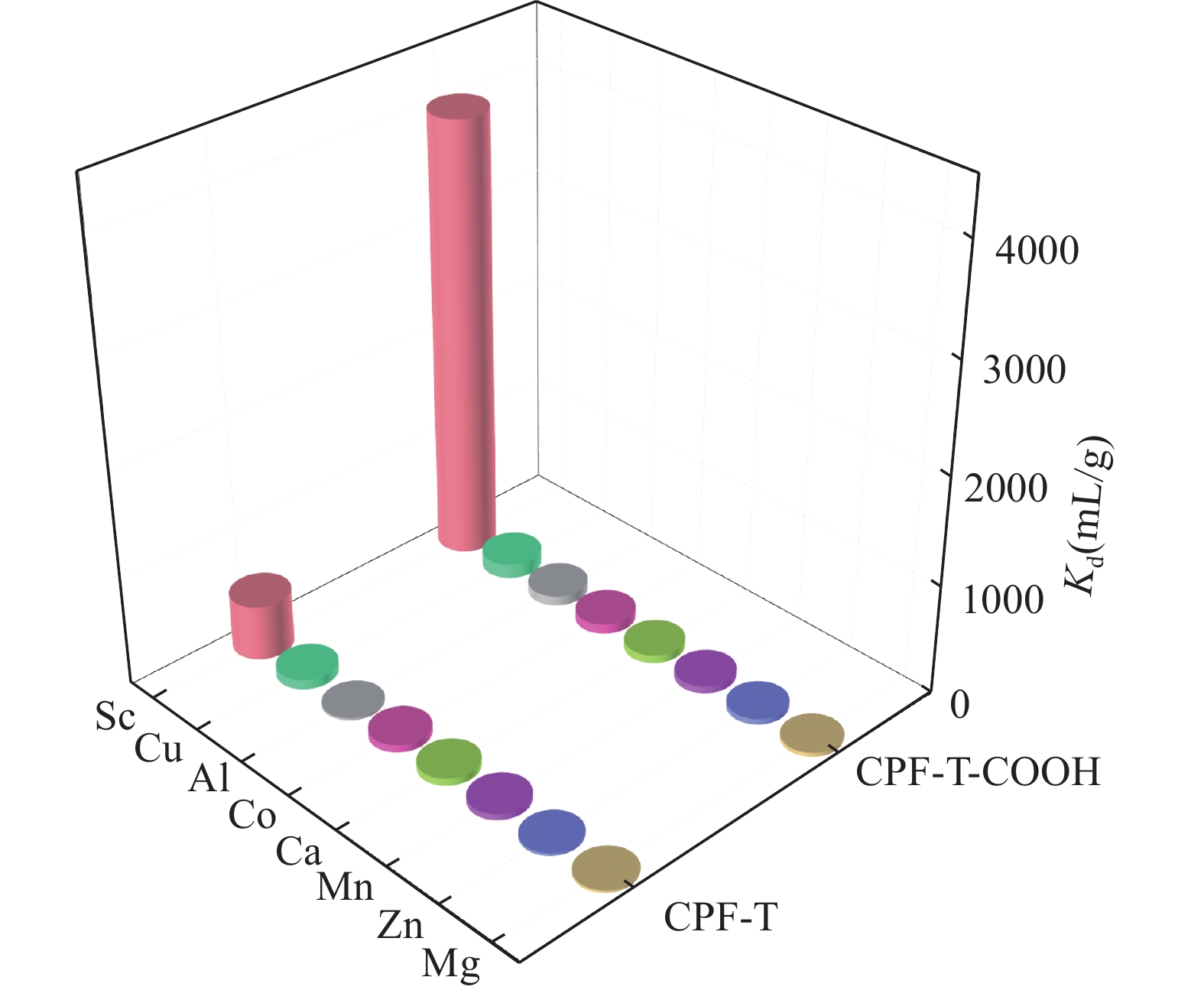
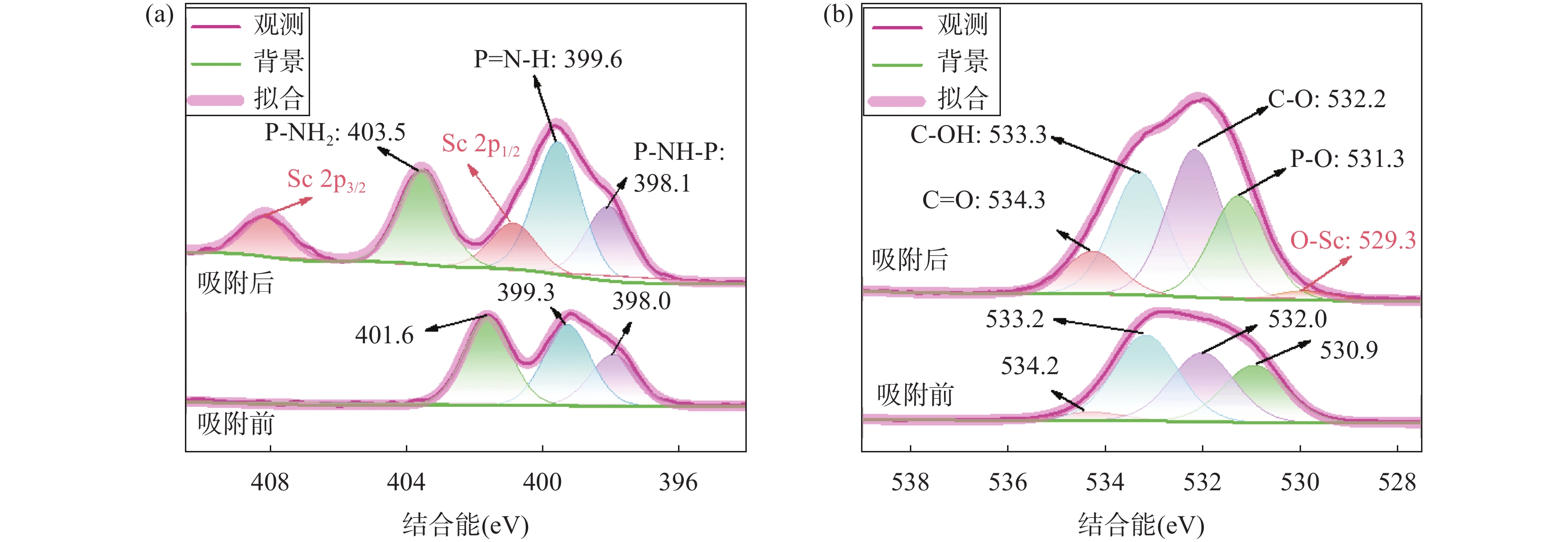
RESULTS CPF-T and CPF-T-COOH were characterized by FT-IR, SEM, TGA and N2 adsorption-desorption analysis. The appearance of P-O-Ar, P=N, and P-N stretching vibration peaks in the FT-IR diagram indicates the successful crosslinking of the organic monomers. In addition, the appearance of C=O, C-O and -OH in CPF-T-COOH indicates the successful modification of —COOH. TGA characterization indicates that the resulting materials have a good thermal stability within 150℃. Through SEM images, it can be observed that the micro morphologies of the two materials are different. CPF-T presents a relatively smooth spherical structure with some agglomerating, CPF-T-COOH exhibits an irregular, ant-like porous structure with a rough surface. The N2 adsorption-desorption isotherms of both materials belong to type II and show a strong absorption in the range of P/P0=0.8−1.0, indicating the presence of microporous and mesoporous structures. The specific surface area of CPF-T is 76.5m2/g, and total pore volume is 0.38cm3/g. The specific surface area of CPF-T-COOH is 2.61m2/g, and the total pore volume is 0.035cm3/g. The adsorption performance of CPF-T and CPF-T-COOH for Sc(III) was investigated through batch adsorption experiments, including the influence of solution pH, adsorption kinetics, adsorption isotherms and adsorption selectivity. The adsorption efficiencies of Sc(III) on the two materials exhibit different trends with respect to the solution pH. As the acidity of the solution increases, the adsorption efficiency of CPF-T for Sc(III) significantly decreases, and at pH<3, the adsorption rate is below 60%. However, within the range of solution pH=1-3, the adsorption efficiency of CPF-T-COOH for Sc(III) remains above 95%. The adsorption kinetics experiment shows that CPF-T reaches adsorption equilibrium within 120min, while CPF-T-COOH can reach adsorption equilibrium within 30min. In addition, in the adsorption isotherms experiment, the maximum adsorption capacity of CPF-T-COOH (64.6mg/g) for Sc(III) was higher than that of CPF-T (22.5mg/g). Finally, the adsorption selectivity was evaluated through coexisting ion experiments. Both materials have the ability to selectively adsorb Sc(III), with Kd values of 5.1×102mL/g and 4.0×103mL/g for Sc(III), respectively. The adsorption performance of CPF-T-COOH is not only higher than that of CPF-T, but also has unique advantages compared to some reported adsorbents, including a wider pH range, fast adsorption equilibrium time, and higher Kf value. These results indicate that the synergistic effect of multiple adsorption sites is an effective strategy for improving adsorption affinity of adsorbents. The adsorption mechanism was studied through XPS analysis. In the high-resolution XPS spectrum of N1s of Sc(III) loaded sample, all the binding energies of P-NH-P(398.0eV), P=N-H(399.3eV) and P-NH2(401.6eV) show an increase, shifting to 398.1eV, 399.6eV and 403.5eV, respectively. Meanwhile, in the spectra of O1s, it can also be observed that the binding energies of C=O(534.2eV), C-OH(533.2eV), C-O(532.0eV) and P-O(531.0eV) shift to 534.3eV, 533.3eV, 532.2eV and 531.3eV, respectively. These results indicate the existence of coordination between O/N and Sc(III). In addition, a new peak at 529.3eV corresponding to O-Sc bond appears in the spectrum of O1s for Sc(III) loading sample, and the peak area of C-OH significantly decreases. This is because the H atom in the hydroxyl group is replaced by Sc(III) through ion exchange, resulting in the formation of a new coordination bond with O.
CONCLUSIONS Covalent phosphonitrile polymer featuring abundant carboxyl functional groups is successfully synthesized by the solvothermal method. Compared with CPF-T, the carboxyl-functionalized CPF-T-COOH exhibits a much stronger binding ability toward Sc(III), where the adsorption efficiency of Sc(III) in acidic media is greatly improved from ~60% to greater than 95%. In addition, its adsorption capacity is 3.5 times that of CPF-T. The result of the mechanism study reveals that the enhanced adsorption performance is attributed to the synergistic effect of multiple functional groups, providing an alternative route for the preparation of new materials with high adsorption performance for Sc(III) capture.
-

-
表 1 CPF-T和CPF-T-COOH吸附Sc (III)的准一级和准二级动力学模型拟合参数
Table 1. The fitting parameters of the pseudo first-order and the pseudo second-order kinetic models for the adsorption of Sc (III) by CPF-T and CPF-T-COOH.
吸附剂 准一级动力学模型 准二级动力学模型 K1
(min−1)qe1
(mg/g)R2 K2
[g/(mg·min)]qe2
(mg/g)R2 CPF-T 0.1475 18.38 0.93 0.01195 19.83 0.98 CPF-T-COOH 0.3693 44.42 0.99 0.02216 46.78 1.0 表 2 CPF-T 和CPF-T-COOH吸附Sc(III)的Langmuir和Freundlich等温线模型拟合参数
Table 2. The fitting parameters of the Langmuir and Freundlich models for the adsorption of Sc(III) by CPF-T and CPF-T-COOH.
吸附剂 Langmuir等温线模型 Freundlich等温线模型 KL
(L/mg)qmax
(mg/g)R2 Kf
[(mg/g)·(mg/L)−1/n]n R2 CPF-T 4.099 22.48 0.93 12.21 5.998 0.74 CPF-T-COOH 5.994 64.63 0.96 49.97 15.74 0.74 表 3 CPF-T-COOH与部分已报道的Sc (III)吸附剂性能 (最佳pH、吸附平衡时间、最大吸附容量、KL值)对比
Table 3. Comparison of Sc (III) adsorption performance (the optimal pH, adsorption equilibrium time, maximum adsorption capacity, and the value of KL) between CPF-T-COOH and some reported adsorbents.
材料名称 溶液
pH吸附平衡时间
(h)最大吸附容量
(mg/g)KL
(mL/g)参考
文献0.075-AA-0.072@MIL-101 4.5 5 90.21 1.7×105 [14] BT/CoFe2O4@SiO2-CMC/PAN 5 5 49.05 2.9×105 [43] IIPBT/CoFe2O4@SiO2 5 2 128 - [51] CLx/SiO2 6 约为1 23.76 - [52] P40-750 3 约为10 18.63 3.1×105 [3] 2-MWNT-sil-P 4 24 32.92 - [53] CPF-T-COOH 2 0.5 64.63 6.0×106 本研究 注:0.075-AA-0.072@MIL-101为丙烯酸功能化的金属有机骨架材料; BT/CoFe2O4@SiO2-CMC/PAN为羧甲基壳聚糖和1-(2-吡啶偶氮)-2-萘基功能化的磁性膨润土纳米材料; IIPBT/CoFe2O4@SiO2为离子印迹纳米复合磁性膨润土; CLx/SiO2为纤维素基二氧化硅纳米复合材料; 2-MWNT-sil-P为碳纳米管和二氧化硅复合材料。
-
[1] Barteková E, Kemp R. National strategies for securing a stable supply of rare earths in different world regions[J]. Resources Policy, 2016, 49: 153−164. doi: 10.1016/j.resourpol.2016.05.003
[2] Williams-Jones A E, Vasyukova O V. The economic geology of scandium, the runt of the rare earth element litter[J]. Economic Geology, 2018, 113(4): 973−988. doi: 10.5382/econgeo.2018.4579
[3] Dai X, Thi Hong Nhung N, Hamza M F, et al. Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar[J]. Separation and Purification Technology, 2022, 292: 121043. doi: 10.1016/j.seppur.2022.121043
[4] Avdibegović D, Regadío M, Binnemans K. Recovery of scandium(III) from diluted aqueous solutions by a supported ionic liquid phase (SILP)[J]. RSC Advances, 2017, 7(78): 49664−49674. doi: 10.1039/C7RA07957E
[5] Yu Q, Ning S, Zhang W, et al. Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent[J]. Hydrometallurgy, 2018, 181: 74−81. doi: 10.1016/j.hydromet.2018.07.025
[6] Lu J, Li S, Tao S, et al. Efficient CO2 electrolysis with scandium doped titanate cathode[J]. International Journal of Hydrogen Energy, 2017, 42(12): 8197−8206. doi: 10.1016/j.ijhydene.2017.01.182
[7] Zhang W, Ning S, Zhang S, et al. Synthesis of functional silica composite resin for the selective separation of zirconium from scandium[J]. Microporous and Mesoporous Materials, 2019, 288: 109602. doi: 10.1016/j.micromeso.2019.109602
[8] 杨波, 杨莉, 孟文祥. 电子探针技术探究钪在白云鄂博矿床不同矿物中的赋存特征[J]. 岩矿测试, 2022, 41(2): 185−198. doi: 10.3969/j.issn.0254-5357.2022.2.ykcs202202005
Yang B, Yang L, Meng W X. Application of electron probe microanalyzer in exploring the occurrence characteristics of scandium in different minerals of the Bayan Obo deposit[J]. Rock and Mineral Analysis, 2022, 41(2): 185−198. doi: 10.3969/j.issn.0254-5357.2022.2.ykcs202202005
[9] Hamza M F, Salih K A M, Abdel-Rahman A A H, et al. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples)[J]. Chemical Engineering Journal, 2021, 403: 126399. doi: 10.1016/j.cej.2020.126399
[10] Rout P C, Sarangi K. A systematic study on extraction and separation of scandium using phosphinic acid by both solvent extraction and hollow fibre membrane[J]. Mineral Processing and Extractive Metallurgy, 2021, 131(2): 166−176.
[11] Liu C, Chen L, Chen J, et al. Application of P507 and isooctanol extraction system in recovery of scandium from simulated red mud leach solution[J]. Journal of Rare Earths, 2019, 37(9): 1002−1008. doi: 10.1016/j.jre.2018.12.004
[12] Ye Q, Li G, Deng B, et al. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204[J]. Separation and Purification Technology, 2019, 209: 175−181. doi: 10.1016/j.seppur.2018.07.033
[13] Chen Y, Ma S, Ning S, et al. Highly efficient recovery and purification of scandium from the waste sulfuric acid solution from titanium dioxide production by solvent extraction[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106226. doi: 10.1016/j.jece.2021.106226
[14] Lou Z N, Xiao X, Huang M N, et al. Acrylic acid-functionalized metal-organic frameworks for Sc(III) selective adsorption[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11772−11781.
[15] Zhang L, Chang X, Zhai Y, et al. Selective solid phase extraction of trace Sc(III) from environmental samples using silica gel modified with 4-(2-morinyldiazenyl)-N-(3-(trimethylsilyl)propyl)benzamide[J]. Analytical Chimica Acta, 2008, 629: 84−91. doi: 10.1016/j.aca.2008.09.039
[16] Garg R, Garg R, Okon Eddy N, et al. Biosynthesized silica-based zinc oxide nanocomposites for the sequestration of heavy metal ions from aqueous solutions[J]. Journal of King Saud University-Science, 2022, 34(4): 101996. doi: 10.1016/j.jksus.2022.101996
[17] Zhao Z, Baba Y, Yoshida W, et al. Development of novel adsorbent bearing aminocarbonylmethylglycine and its application to scandium separation[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(11): 2779−2784.
[18] Zhao R, Liao H, Wu X, et al. Selective adsorption behaviours of MOFs@SiO2 with different pore sizes and shell thicknesses[J]. Journal of Solid State Chemistry, 2020, 292: 121693. doi: 10.1016/j.jssc.2020.121693
[19] Liu Z, Li H. Metallurgical process for valuable elements recovery from red mud—A review[J]. Hydrometallurgy, 2015, 155: 29−43. doi: 10.1016/j.hydromet.2015.03.018
[20] Borra C R, Blanpain B, Pontikes Y, et al. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review[J]. Journal of Sustainable Metallurgy, 2016, 2(4): 365−386. doi: 10.1007/s40831-016-0068-2
[21] Mines P D, Thirion D, Uthuppu B, et al. Covalent organic polymer functionalization of activated carbon surfaces through acyl chloride for environmental clean-up[J]. Chemical Engineering Journal, 2017, 309: 766−771. doi: 10.1016/j.cej.2016.10.085
[22] Fan L, Duan H L, Wang J, et al. Preparation of fluorinated covalent organic polymers at room temperature for removal and detection of perfluorinated compounds[J]. Journal of Hazardous Materials, 2021, 420: 126659. doi: 10.1016/j.jhazmat.2021.126659
[23] Ravi S, Kim S Y, Bae Y S. Novel benzylphosphate-based covalent porous organic polymers for the effective capture of rare earth elements from aqueous solutions[J]. Journal of Hazardous Materials, 2022, 424: 127356. doi: 10.1016/j.jhazmat.2021.127356
[24] Zhang M, Li Y, Bai C, et al. Synthesis of microporous covalent phosphazene-based frameworks for selective separation of uranium in highly acidic media based on size-matching effect[J]. ACS Applied Material & Interfaces, 2018, 10(34): 28936−28947.
[25] Li X, Qi Y, Yue G, et al. Solvent- and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(Ⅵ) and Hg(II)[J]. Green Chemistry, 2019, 21(3): 649−657. doi: 10.1039/C8GC03295E
[26] Huang J, Cui W R, Wang Y G, et al. Rational designed molecularly imprinted triazine-based porous aromatic frameworks for enhanced palladium capture via three synergistic mechanisms[J]. Chemical Engineering Journal, 2022, 430: 132962. doi: 10.1016/j.cej.2021.132962
[27] Zhang W, Wang S, Cao X, et al. A facile coordinate complexing of Na(I) to fabricate SPB-ACE@MS-4A for selective adsorption to monovalent alkali metal ions[J]. Desalination, 2021, 520: 115337. doi: 10.1016/j.desal.2021.115337
[28] Huang L J, Shen R J, Liu R Q, et al. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water[J]. Journal of Hazardous Materials, 2020, 392: 122320. doi: 10.1016/j.jhazmat.2020.122320
[29] 周慧君, 帅琴, 黄云杰, 等. 双硫腙改性氧化石墨烯/壳聚糖复合微球固相萃取在线富集-原子荧光光谱法测定地质样品中痕量汞[J]. 岩矿测试, 2017, 36(5): 474−480.
Zhou H J, Shuai Q, Huang Y J, et al. On-line determination of Hg(II) in geological samples by afs after solid phase extraction using dithizone-modified graphene oxide/chitosan composite microspheres[J]. Rock and Mineral Analysis, 2017, 36(5): 474−480.
[30] Ravi S, Puthiaraj P, Yu K, et al. Porous covalent organic polymers comprising a phosphite skeleton for aqueous Nd(III) capture[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11488−11497.
[31] 赵亮, 李嘉权, 王岩, 等. 氨基改性Cu-BTC对水溶液中镧铈稀土离子的吸附研究[J]. 石油化工, 2023, 52(4): 469−477. doi: 10.3969/j.issn.1000-8144.2023.04.005
Zhao L, Li J Q, Wang Y, et al. Adsorption of rare earth ions la and ce from aqueous solution by amino-modified Cu-BTC[J]. Petrochemical Technology, 2023, 52(4): 469−477. doi: 10.3969/j.issn.1000-8144.2023.04.005
[32] Xie Y, Wu Y, Liu X, et al. Rational design of cooperative chelating sites on covalent organic frameworks for highly selective uranium extraction from seawater[J]. Cell Reports Physical Science, 2023, 4(1): 101220. doi: 10.1016/j.xcrp.2022.101220
[33] Liu M, Chen J, Zou D, et al. A novel synergistic extraction system for the recovery of scandium(III) from sulfuric acid medium with mixed cyanex923 and N1923[J]. Separation and Purification Technology, 2022, 283: 120223. doi: 10.1016/j.seppur.2021.120223
[34] Hu Z P, Jiang H L, Hu Q D, et al. Preparation of the hexachlorocyclotriphosphazene crosslinked sodium alginate polymer/multi-walled carbon nanotubes composite powder for the removal of the cationic dyes[J]. Journal of Molecular Structure, 2022, 1262: 133050. doi: 10.1016/j.molstruc.2022.133050
[35] Lv M, Yao C, Yang D, et al. Synthesis of a melamine-cyclotriphosphazene derivative and its application as flame retardant on cotton gauze[J]. Journal of Applied Polymer Science, 2016, 133(25): 43555.
[36] Mohamed M G, Hsiao C H, Luo F, et al. Multifunctional polybenzoxazine nanocomposites containing photoresponsive azobenzene units, catalytic carboxylic acid groups, and pyrene units capable of dispersing carbon nanotubes[J]. RSC Advances, 2015, 5(56): 45201−45212. doi: 10.1039/C5RA07983G
[37] Liu R, Yan Q, Tang Y, et al. Nacl Template-assisted synthesis of self-floating COFs foams for the efficient removal of sulfamerazine[J]. Journal of Hazardous Materials, 2022, 421: 126702. doi: 10.1016/j.jhazmat.2021.126702
[38] Xu S, Weng Z, Tan J, et al. Hierarchically structured porous organic polymer microspheres with built-in Fe3O4 supraparticles: Construction of dual-level pores for Pt-catalyzed enantioselective hydrogenation[J]. Polymer Chemistry, 2015, 6(15): 2892−2899. doi: 10.1039/C4PY01611D
[39] Ma Z, Liu F, Liu N, et al. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg(II) removal from water[J]. Journal of Hazardous Materials, 2021, 405: 124190. doi: 10.1016/j.jhazmat.2020.124190
[40] Chen Y, Wang S, Li Y, et al. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase[J]. Journal of Colloid and Interface Science, 2020, 575: 367−376. doi: 10.1016/j.jcis.2020.04.110
[41] Wang P, Ding F, Huang Z, et al. Adsorption behavior and mechanism of Cd(II) by modified coal-based humin[J]. Environmental Technology & Innovation, 2021, 23: 101699.
[42] Wood S A, Samson I M. The aqueous geochemistry of gallium, germanium, indium and scandium[J]. Ore Geology Reviews, 2006, 28(1): 57−102. doi: 10.1016/j.oregeorev.2003.06.002
[43] Zhang L, Wang C, Yang R, et al. Novel environment-friendly magnetic bentonite nanomaterials functionalized by carboxymethyl chitosan and 1-(2-pyridinylazo)-2-naphthaleno for adsorption of Sc(III)[J]. Applied Surface Science, 2021, 566: 150644. doi: 10.1016/j.apsusc.2021.150644
[44] Salman A D, Juzsakova T, Akos R, et al. Synthesis and surface modification of magnetic Fe3O4@SiO2 core-shell nanoparticles and its application in uptake of scandium(III) ions from aqueous media[J]. Environmental Science Pollution Research, 2021, 28(22): 28428−28443. doi: 10.1007/s11356-020-12170-4
[45] Wang J, Guo X. Adsorption kinetic models: Physical meanings, applications, and solving methods[J]. Journal of Hazardous Materials, 2020, 390: 122156. doi: 10.1016/j.jhazmat.2020.122156
[46] Xu W, Zhou S, Wang B, et al. Efficient adsorption of Au(III) from acidic solution by a novel N, S-containing metal–organic framework[J]. Separation and Purification Technology, 2022, 288: 120646. doi: 10.1016/j.seppur.2022.120646
[47] 杨梦楠, 孙晗, 曹海龙, 等. 生物炭-壳聚糖磁性复合吸附的制备及去除地下水中铅和铜[J]. 岩矿测试, 2023, 42(3): 563−575.
Yang M N, Sun H, Cao H L, et al. Preparation and application of biochar-chitosan magnetic composite adsorbent for removal of lead and copper from groundwater[J]. Rock and Mineral Analysis, 2023, 42(3): 563−575.
[48] Wang J, Guo X. Adsorption isotherm models: Classification, physical meaning, application and solving method[J]. Chemosphere, 2020, 258: 127279. doi: 10.1016/j.chemosphere.2020.127279
[49] Li X, Liu Z, Wei L, et al. Comparison of competitive and synergistic adsorption of tetrabromobisphenol-A and its metabolites on two different organic-modified clays[J]. Journal of Chemical & Engineering Data, 2019, 64(6): 2780−2790.
[50] Tran H N, Bonilla-Petriciolet A. Improper estimation of thermodynamic parameters in adsorption studies with distribution coefficient Kd (Qe/Ce) or Freundlich constant ( Kf): Considerations from the derivation of dimensionless thermodynamic equilibrium constant and suggestions[J]. Adsorption Science & Technology, 2022, 2022: 1−23.
[51] Wang C, Zhang L, Zhou G, et al. Synthesis of environmental-friendly ion-imprinted magnetic nanocomposite bentonite for selective recovery of aqueous Sc(III)[J]. Journal of Colloid and Interface Science, 2023, 630: 738−750. doi: 10.1016/j.jcis.2022.10.161
[52] Iftekhar S, Srivastava V, Sillanpää M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite[J]. Chemical Engineering Journal, 2017, 320: 151−159. doi: 10.1016/j.cej.2017.03.051
[53] Ramasamy D L, Puhakka V, Doshi B, et al. Fabrication of carbon nanotubes reinforced silica composites with improved rare earth elements adsorption performance[J]. Chemical Engineering Journal, 2019, 365: 291−304. doi: 10.1016/j.cej.2019.02.057
[54] Wu J S, Li Z, Tan H X, et al. Highly selective separation of rare earth elements by Zn-Btc metal-organic framework/nanoporous graphene via in situ green synthesis[J]. Analytical Chemistry, 2021, 93(3): 1732−1739. doi: 10.1021/acs.analchem.0c04407
[55] 王哲, 朱俊, 李雯, 等. 镧沸石对磷和重金属的吸附与底泥钝化性能[J]. 环境科学, 2022, 43(11): 5106−5114.
Wang Z, Zhu J, Li W, et al. Adsorption of phosphate and heavy metals by lanthanum modified zeolite and its performance in sediment inactivation[J]. Environmental Science, 2022, 43(11): 5106−5114.
[56] He J, Lu Y, Luo G. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions[J]. Chemical Engineering Journal, 2014, 244: 202−208. doi: 10.1016/j.cej.2014.01.096
[57] Gao X, Zhang Y, Zhao Y. Biosorption and reduction of Au(III) to gold nanoparticles by thiourea modified alginate[J]. Carbohydrate Polymers, 2017, 159: 108−115. doi: 10.1016/j.carbpol.2016.11.095
-



 下载:
下载: