A study of the hydrochemical characteristics and geothermal water of typical granite geothermal reservoir in the Jiaodong area
-
摘要:
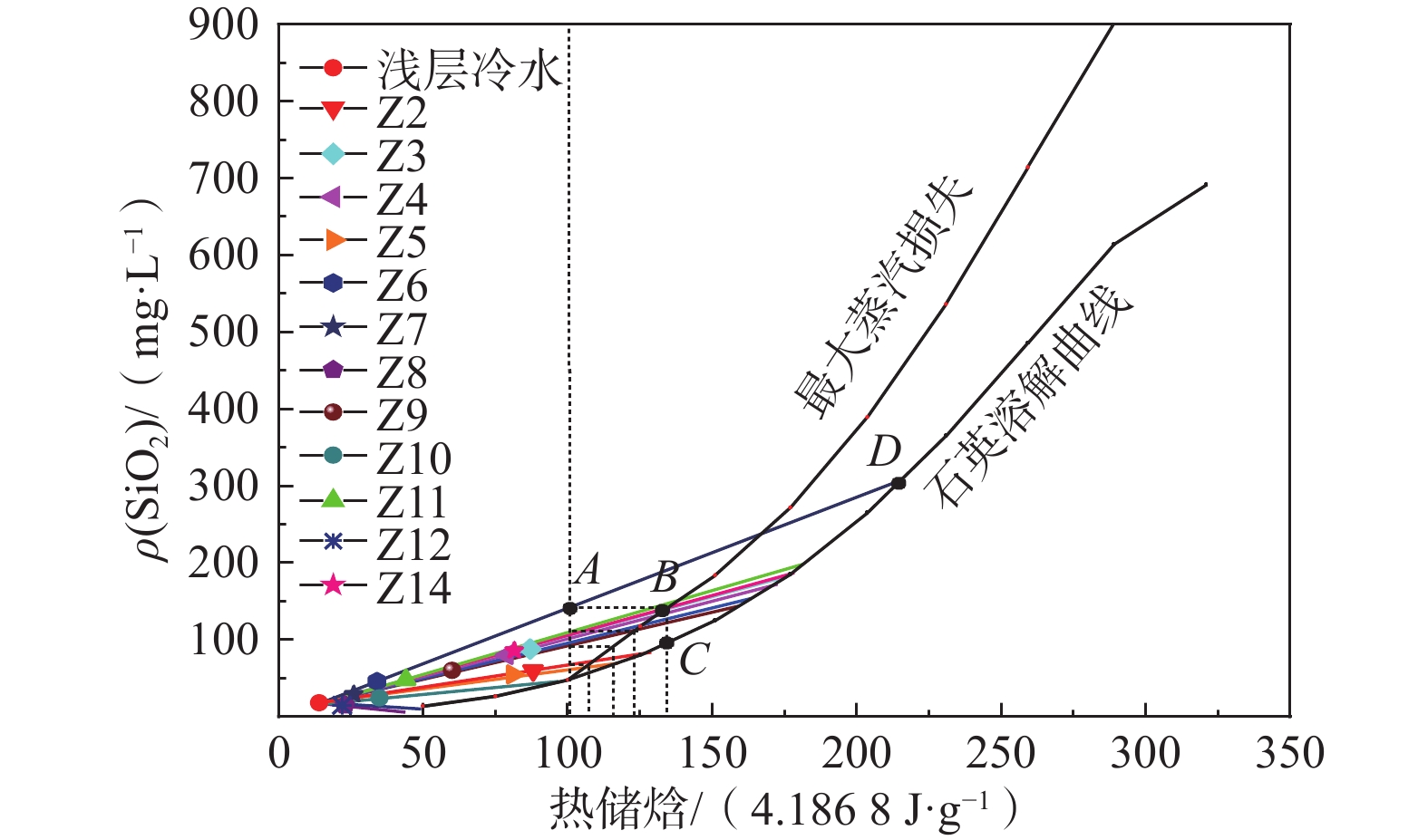
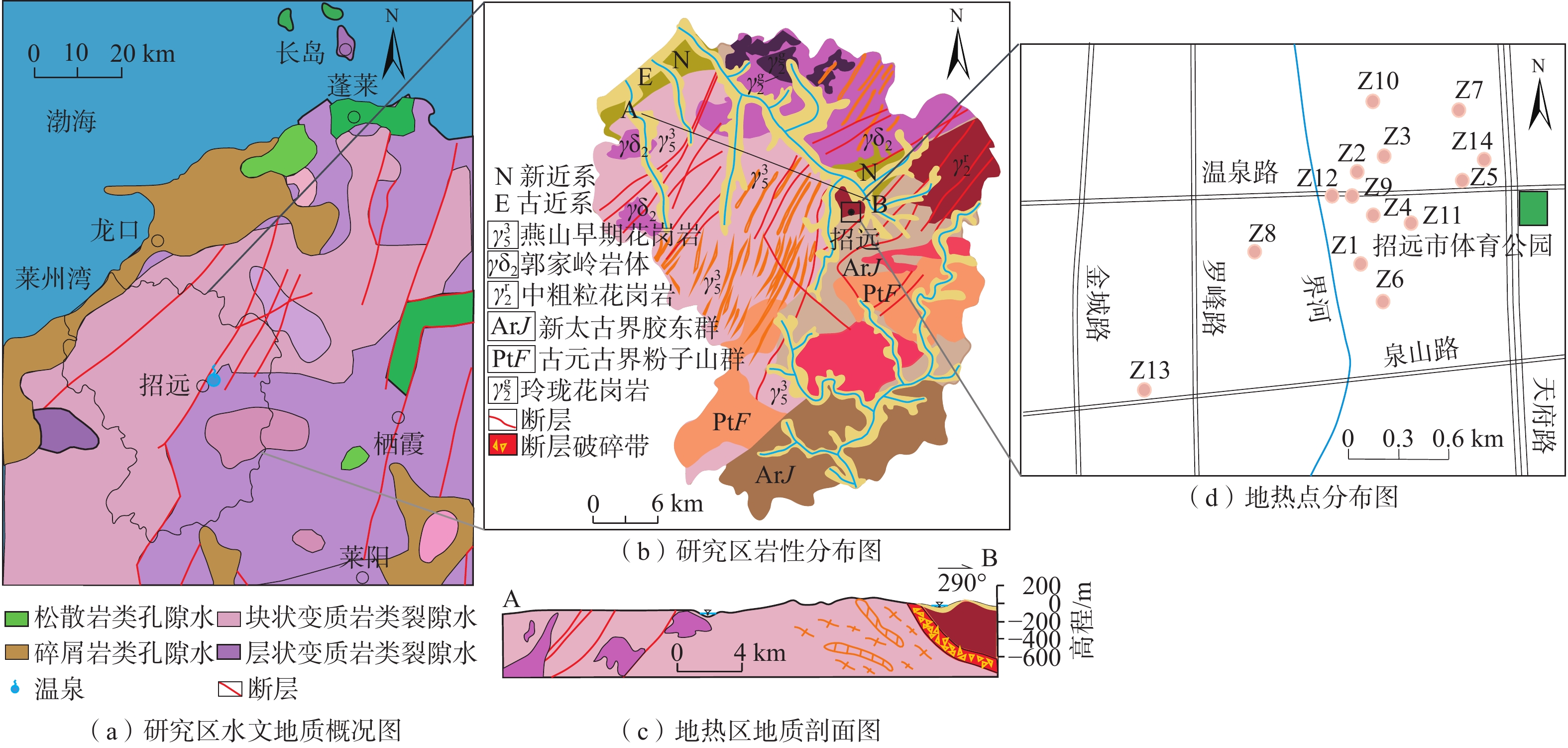
招远地热田位于胶东隆起区,元古代蚀变花岗岩分布广泛,地下热水微量元素丰富。为查明地下热水微量组分的赋存条件、花岗岩热储环境与地热资源量,利用地下热水水化学分析、热储分析及有效能源换算法,建立Gibbs模型,进行PHREEQC模拟并开展热储估算。研究结果显示:(1)地下热水水化学类型为Cl—Na型,与海水水化学类型一致,地下热水溶解性固体总量(TDS)介于1359.7~5302.0 mg/L,锶、溴、偏硅酸等微量组分的质量浓度分别达26.20,7.50,88.00 mg/L,均超过国家医疗热矿水水质标准;(2)地热田东北方向的玲珑花岗岩中锶的质量分数较高,介于334~1 805 mg/kg,是地下热水中锶的一个重要来源;(3)热储温度在107~215 °C之间,硅-焓图解法分析冷水混入比例为33.6%~58.9%。结果显示:40~60 °C的总可用能源19.73 TJ/a,总热能达5479.57 MW·h,吨油当量471.16 toe;>60 °C的总可用能源301.57 TJ/a,总热能达83771.53 MW·h,吨油当量为7203.06 toe。综合分析认为研究区地热资源丰富,地下热水微量组分来源于花岗岩热储层的溶滤作用,富集过程受热储环境的影响,研究结果有助于完善地下热水水-岩相互作用理论。
Abstract:Zhaoyuan is located in Jiaodong uplift zone. Proterozoic altered granites are widely distributed. The trace elements are enriched in geothermal fields. In order to ascertain the occurrence conditions of the trace components, the granite geothermal reservoir and geothermal resources. We use hydrochemical analysis, reservoir temperature analysis and effective energy conversion methods, establish Gibbs model, PHREEQC model, reservoir estimation, and obtain the occurrence conditions and reservoir environment of trace components of thermal water. The results show that (1) Zhaoyuan geothermal water, similar to seawater, is Cl—Na type. The total dissolved solids (TDS) of geothermal water ranges from 1359.7 mg/L to 5302.0 mg/L, and the mass concentrations of strontium, bromine and metasilicic acid are 26.20, 7.50 and 88.00 mg/L, respectively, which exceed the national medical thermal mineral water quality standards. (2) The Linglong granite in the northeast of the geothermal field has high strontium content, ranging from 334 mg/kg to 1 805 mg/kg, which is an important source of strontium. (3) The reservoir temperature of geothermal field is between 107 °C and 215 °C, and the mixing proportion of cold water is between 33.6% and 58.9% calculated from silicon enthalpy diagram. The calculation results indicate that available energy is 19.73 TJ/a, 5479.57 MW·h and 471.16 toe between 40 and 60 °C. The total available energy is 301.57 TJ/a, 83771.53 MW·h, and 7203.06 toe over 60 °C. The trace components come from lixiviation between strontium-rich granite surrounding rock and groundwater. The enrichment process of trace elements is affected by geothermal reservoir. This study may provide a better understanding of the characteristics of geothermal water in the granite thermal reservoirs, and enrich and promote the theory of geothermal water-rock interactions.
-
Key words:
- geothermal water /
- trace components /
- reservoir temperature /
- geothermal energy /
- water-rock interaction
-

-
表 1 研究区地下热水组分质量浓度、TDS、碱度及计算获得的
$P_{\rm{CO_2}} $ Table 1. Mass concentration of constituents, TDS, alkalinity and calculated
$P_{\rm{CO_2}} $ of 15 samples水样 ρ(  )
)
/(mg·L−1)ρ 
/(mg·L−1)ρ(  )
)
/(mg·L−1)ρ(  )
)
/(mg·L−1)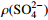
/(mg·L−1)
/(mg·L−1)ρ(SiO2)
/(mg·L−1)TDS
/(mg·L−1)碱度
/(mg·kg−1 CaCO3)电荷平衡
/%
/PaS1 11100.0 1150.0 353.0 19400.0 2280.0 104.0 0.6 34800.0 109.0 1.06 69 Z1 1575.8 10.1 240.9 2664.8 99.6 252.2 — 4717.3 200.3 0.15 9300 Z2 1618.1 5.1 231.4 2731.8 123.4 149.4 60.0 4853.9 128.1 0.03 730 Z3 1748.7 12.5 251.6 2990.1 125.7 146.3 88.0 5302.0 113.4 0.11 1400 Z4 1731.4 1.8 253.1 2922.1 116.4 186.7 80.0 5206.9 156.5 0.01 1300 Z5 1343.1 9.3 224.8 2236.3 119.8 281.8 55.0 4136.4 200.5 0.46 3800 Z6 455.3 3.9 55.2 587.7 52.8 308.7 46.0 1359.7 253.1 0.03 580 Z7 1449.6 35.7 270.9 2596.0 80.4 275.8 28.8 4603.2 223.2 0.05 560 Z8 968.2 63.1 189.9 1765.3 93.4 291.9 15.0 3256.6 248.2 0.02 1400 Z9 1647.5 11.4 238.4 2788.2 112.1 202.0 60.0 4970.1 167.3 0.02 630 Z10 1766.5 16.6 251.1 2961.1 122.2 266.8 24.0 5286.3 222.0 0.01 750 Z11 773.6 8.4 74.9 1144.1 76.2 242.0 48.0 2253.4 204.0 0.14 270 Z12 1398.7 26.3 218.9 2360.6 94.0 316.1 15.0 4277.8 268.4 0.03 1900 Z13 100.6 56.2 98.9 1155.9 494.6 313.0 — 2172.6 252.7 4.80 1800 Z14 1188.0 4.2 162.1 1924.7 346.8 224.1 85.7 4027.5 181.9 1.80 1200 表 2 Z14热水点矿物的SI
Table 2. SI of minerals of sample Z14
矿物名称 SI 矿物名称 SI 矿物名称 SI 钠长石 −1.52 温石棉 −1.86 羟基磷灰石 1.28 硬石膏 −0.83 CO2(g) −1.55 伊利石 −2.22 钙长石 −2.88 白云石 0.03 钾长石 −0.96 文石 0.54 毒重石 −2.81 云母 2.87 重晶石 0.40 Fe(OH)3(a) −0.16 高岭石 −0.47 钙蒙脱石 −2.05 萤石 −0.01 石英 0.33 方解石 0.65 水铝矿 −1.07 菱锰矿 −0.11 天青石 0.00 石膏 −1.09 锶长石 0.12 玉髓 0.04 石盐 −4.38 滑石 2.52 绿泥石 −0.26 锰矿 −1.35 表 3 硅-焓法计算获得的参数
Table 3. Parameters calculated with the silicon enthalpy method
热水 温度/℃ 考虑蒸汽损失
的热储温度/℃不考虑蒸汽损
失热储温度/℃蒸汽损
失量/%冷水
比例/%热储
温度/℃Z2 88 107.23 126.00 18.5 33.6 125.73 Z3 87 119.11 171.33 28.5 53.9 172.65 Z4 79 119.87 170.12 29.6 58.9 172.25 Z5 81 106.40 123.96 15.2 39.3 125.33 Z6 34 134.17 217.11 35.5 90.2 215.71 Z7 26 119.49 170.57 28.1 92.2 167.42 Z9 60 117.45 164.44 27.7 70.0 167.90 Z10 35 — 92.10 — 74.4 97.08 Z11 44 121.91 180.34 32.6 81.6 176.60 Z14 82 121.54 173.83 29.7 58.6 176.11 表 4 地下热水点基本参数及地热能指标
Table 4. Parameters of the geothermal water and geothermal energy indexes
水样点 井深/m 水头/m Favg~Fmax/(kg·s–1) Ti/℃ To/℃ C/MWt E/(TJ·a–1) Z1 347.0 65.9 3.9~5.1 64 20 0.94 26.16 Z2 218.1 — 3.9~8.9 88 20 2.52 57.21 Z3 252.5 — 9.7~30.0 92 83 1.13 23.58 Z4 400.0 61.2 17.2~26.3 79 75 0.44 11.47 Z5 400.0 66.3 3.7~4.9 100 81 0.40 11.03 Z6 280.0 65.9 6.7~13.2 46 34 0.67 15.78 Z7 200.0 59.3 0.8~0.8 32 26 0.02 0.62 Z8 223.7 66.2 0.5~0.5 23 20 0.01 0.18 Z9 280.4 62.4 1.8~3.7 60 54 0.09 2.18 Z10 226.6 61.4 1.2~1.3 35 33 0.01 0.34 Z11 360.0 62.8 0.8~1.5 44 32 0.07 1.77 Z12 270.1 59.9 1.1~1.2 22 18 0.02 0.60 Z13 81.8 39.8 2.8~2.8 99 20 0.92 29.07 Z14 248.0 63.1 20.8~20.8 82 30 4.54 143.05 表 5 招远温泉不同温度范围地热能潜力
Table 5. Geothermal potential of the hot springs in various temperature ranges in the Zhaoyuan area
温度/℃ C/MWt E/(TJ·a–1) A 热能换算电能/(MW·h) 吨油当量/toe <40 0.06 1.74 0.98 482.02 41.45 40~60 0.83 19.73 0.75 5479.57 471.16 >60 10.89 301.57 0.88 83771.53 7203.06 -
[1] 沈照理, 王焰新, 郭华明. 水-岩相互作用研究的机遇与挑战[J]. 地球科学,2012,37(2):207 − 219. [SHEN Zhaoli, WANG Yanxin, GUO Huaming. Opportunities and challenges of water-rock interaction studies[J]. Earth Science,2012,37(2):207 − 219. (in Chinese with English abstract)
[2] 周训, 金晓媚, 梁四海, 等. 地下水科学专论[M]. 2版. 北京: 地质出版社, 2010
ZHOU Xun, JIN Xiaomei, LIANG Sihai, et al. Monographs on groundwater science[M]. 2nd ed. Beijing: Geological Publishing House, 2010. (in Chinese)
[3] 文冬光, 沈照理, 钟佐燊. 水-岩相互作用的地球化学模拟理论及应用[M]. 武汉: 中国地质大学出版社, 1998
WEN Dongguang, SHEN Zhaoli, ZHONG Zuoyan. Geochemical simulation theory and application of water-rock interaction[M]. Wuhan: China University of Geosciences Press, 1998. (in Chinese)
[4] 王贵玲, 蔺文静. 我国主要水热型地热系统形成机制与成因模式[J]. 地质学报,2020,94(7):1923 − 1937. [WANG Guiling, LIN Wenjing. Main hydro-geothermal systems and their genetic models in China[J]. Acta Geologica Sinica,2020,94(7):1923 − 1937. (in Chinese with English abstract) doi: 10.3969/j.issn.0001-5717.2020.07.002
[5] FOURNIER R O, POTTER II R W. Magnesium correction for the Na-K-Ca chemical geothermometer[J]. Geochimica et Cosmochimica Acta, 1979, 43(9), 1543 − 1550.
[6] GIGGENBACH W F. Geothermal solute equilibria. derivation of Na-K-Mg-Ca geoindicators[J]. Geochimica et Cosmochimica Acta,1988,52(12):2749 − 2765. doi: 10.1016/0016-7037(88)90143-3
[7] FOURNIER R O. Chemical geothermometers and mixing models for geothermal systems[J]. Geothermics,1977,5(1/2/3/4):41 − 50. doi: 10.1016/0375-6505(77)90007-4
[8] FOURNIER R O, ROWE J J. Estimation of underground temperatures from the silica content of water from hot springs and wet-steam wells[J]. American Journal of Science,1966,264(9):685 − 697. doi: 10.2475/ajs.264.9.685
[9] LIU Z H, DREYBRODT W. Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land[J]. Science Bulletin,2015,60(2):182 − 191. doi: 10.1007/s11434-014-0682-y
[10] 高宗军, 孙智杰, 杨永红, 等. 山东省地热水水化学研究及赋存特征[J]. 科学技术与工程,2019,19(20):85 − 90. [GAO Zongjun, SUN Zhijie, YANG Yonghong, et al. Occurrence characteristics and hydrochemical characteristics of geothermal water in Shandong Province[J]. Science Technology and Engineering,2019,19(20):85 − 90. (in Chinese with English abstract) doi: 10.3969/j.issn.1671-1815.2019.20.012
[11] HAO Y L, PANG Z H, KONG Y L, et al. Chemical and isotopic constraints on the origin of saline waters from a hot spring in the eastern coastal area of China[J]. Hydrogeology Journal,2020,28(7):2457 − 2475. doi: 10.1007/s10040-020-02199-7
[12] 孟庆晗, 王鑫, 邢立亭, 等. 济南四大泉群补给来源差异性研究[J]. 水文地质工程地质,2020,47(1):37 − 45. [MENG Qinghan, WANG Xin, XING Liting, et al. A study of the difference in supply sources of the four groups of springs in Jinan[J]. Hydrogeology & Engineering Geology,2020,47(1):37 − 45. (in Chinese with English abstract)
[13] 梁永平, 张发旺, 申豪勇, 等. 山西太原晋祠—兰村泉水复流的岩溶水文地质条件新认识[J]. 水文地质工程地质,2019,46(1):11 − 18. [LIANG Yongping, ZHANG Fawang, SHEN Haoyong, et al. Recognition of the critical hydrogeological conditions of the Jinci Spring and Lancun Spring in Shanxi[J]. Hydrogeology & Engineering Geology,2019,46(1):11 − 18. (in Chinese with English abstract)
[14] 闫佰忠, 邱淑伟, 肖长来, 等. 长白山玄武岩区主要断裂与地热水异常关系[J]. 水文地质工程地质,2017,44(4):34 − 40. [YAN Baizhong, QIU Shuwei, XIAO Changlai, et al. Relationship between the main faults and geothermal water anomaly in the Changbai Mountain basalt area[J]. Hydrogeology & Engineering Geology,2017,44(4):34 − 40. (in Chinese with English abstract)
[15] 金秉福, 张云吉, 栾光忠. 胶东半岛温泉的地热特征[J]. 水文地质工程地质,2000,27(5):31 − 33. [JIN Bingfu, ZHANG Yunji, LUAN Guangzhong. Geothermal characteristics of warm spring in Jiaodong Peninsula[J]. Hydrogeology & Engineering Geology,2000,27(5):31 − 33. (in Chinese with English abstract) doi: 10.3969/j.issn.1000-3665.2000.05.010
[16] 苏春田, 聂发运, 邹胜章, 等. 湖南新田富锶地下水水化学特征与成因分析[J]. 现代地质,2018,32(3):554 − 564. [SU Chuntian, NIE Fayun, ZOU Shengzhang, et al. Hydrochemical characteristics and formation mechanism of strontium-rich groundwater in Xintian County, Hunan Province[J]. Geoscience,2018,32(3):554 − 564. (in Chinese with English abstract)
[17] XU T F, SONNENTHAL E, SPYCHER N, et al. TOUGHREACT— A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration[J]. Computers & Geosciences, 2006, 32(2): 145 − 165.
[18] GIBBS R J. Mechanisms controlling world water chemistry[J]. Science,1970,170(3962):1088 − 1090. doi: 10.1126/science.170.3962.1088
[19] 杨楠, 苏春利, 曾邯斌, 等. 基于水化学和氢氧同位素的兴隆县地下水演化过程研究[J]. 水文地质工程地质,2020,47(6):154 − 162. [YANG Nan, SU Chunli, ZENG Hanbin, et al. Evolutional processes of groundwater in Xinglong County based on hydrochemistry and hydrogen and oxygen isotopes[J]. Hydrogeology & Engineering Geology,2020,47(6):154 − 162. (in Chinese with English abstract)
[20] HU S Y, XIAO C L, LIANG X J, et al. Influence of water-rock interaction on the pH and heavy metals content of groundwater during in situ oil shale exploitation[J]. Oil Shale,2020,37(2):104-118. doi: 10.3176/oil.2020.2.02
[21] 林聪业, 孙占学, 高柏, 等. 拉萨地区地下水水化学特征及形成机制研究[J]. 地学前缘,2021,28(5):49 − 58. [LIN Congye, SUN Zhanxue, GAO Bai, et al. Hydrochemical characteristics and formation mechanism of groundwater in Lhasa area, China[J]. Earth Science Frontiers,2021,28(5):49 − 58. (in Chinese with English abstract)
[22] WANG X C, ZHOU X, ZHAO J B, et al. Hydrochemical evolution and reaction simulation of travertine deposition of the Lianchangping hot springs in Yunnan, China[J]. Quaternary International,2015,374:62 − 75. doi: 10.1016/j.quaint.2014.09.046
[23] WANG X C, ZHOU X. Geothermometry and circulation behavior of the hot springs in Yunlong County of Yunnan in southwest China[J]. Geofluids,2019,2019:8432496.
[24] FOURNIER R O. Geochemical and hydrologic considerations and the use of enthalpy-chloride diagrams in the prediction of underground conditions in hot-spring systems[J]. Journal of Volcanology and Geothermal Research,1979,5(1/2):1 − 16. doi: 10.1016/0377-0273(79)90029-5
[25] FOURNIER R O. Silica in thermal waters: laboratory and field investigations[C]//Proceedings of international symposium on hydrogeochemistry and biogeochemistry. Tokyo, 1973: 132 − 139.
[26] FOURNIER R O, POTTER II R W. An equation correlating the solubility of quartz in water from 25 °C to 900 °C at pressures up to 10 000 bars[J]. Geochimica et Cosmochimica Acta,1982,46(10):1969 − 1973. doi: 10.1016/0016-7037(82)90135-1
[27] HENLEY R W, ELLIS A J. Geothermal systems ancient and modern: A geochemical review[J]. Earth-Science Reviews,1983,19(1):1 − 50. doi: 10.1016/0012-8252(83)90075-2
[28] LUND J W, FREESTON D H. World-wide direct uses of geothermal energy 2000[J]. Geothermics,2001,30(1):29 − 68. doi: 10.1016/S0375-6505(00)00044-4
[29] JOKSIMOVIĆ M, PAVLOVIĆ M A. Conditions and possibilities of direct utilisation of thermal-mineral waters in Raska region, Serbia[J]. Renewable and Sustainable Energy Reviews,2014,32:107 − 113. doi: 10.1016/j.rser.2013.12.048
[30] RISTIĆ D, VUKOIČIĆ D, NIKOLIĆ M, et al. Capacities and energy potential of thermal-mineral springs in the area of the Kopaonik tourist region (Serbia)[J]. Renewable and Sustainable Energy Reviews,2019,102:129 − 138. doi: 10.1016/j.rser.2018.12.005
[31] MIRANDA M M, MATOS C R, RODRIGUES N V, et al. Effect of temperature on the thermal conductivity of a granite with high heat production from Central Portugal[J]. Journal of Iberian Geology,2019,45(1):147 − 161. doi: 10.1007/s41513-018-0096-9
-



 下载:
下载: