Study on the remediation effect and influencing factors of stabilized biochar supported with nano zero-valent iron on Cr(VI) in groundwater
-
摘要:
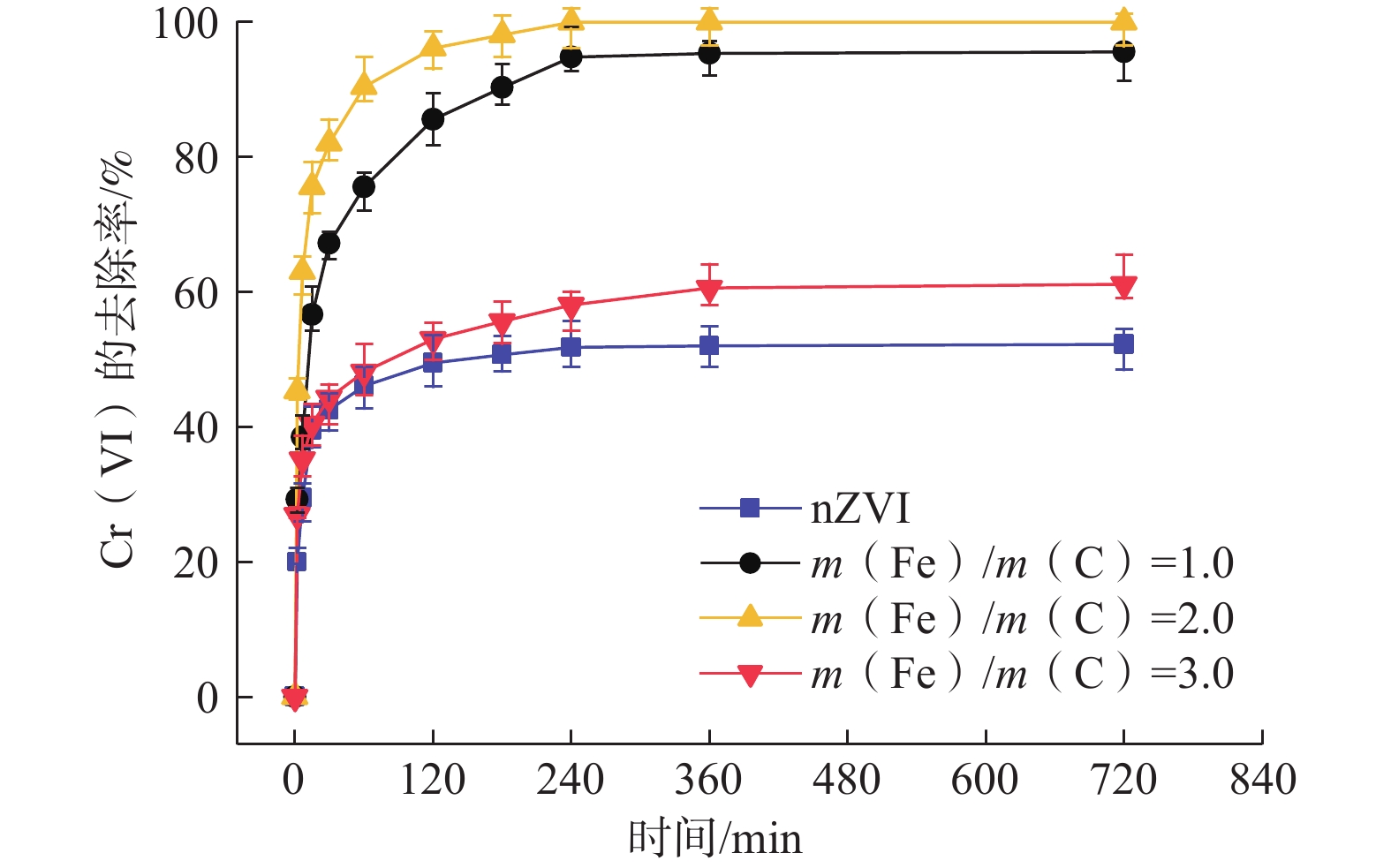
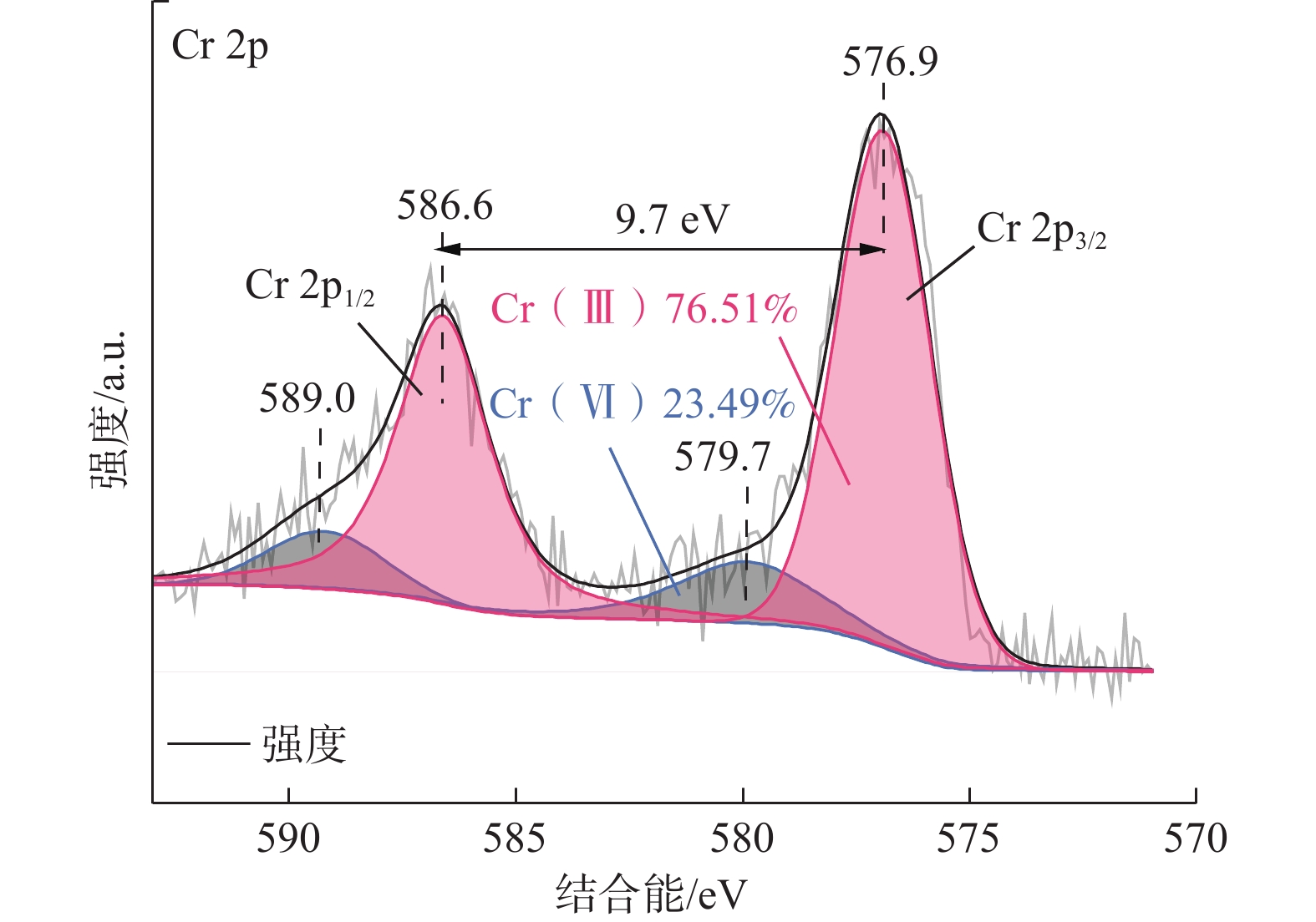
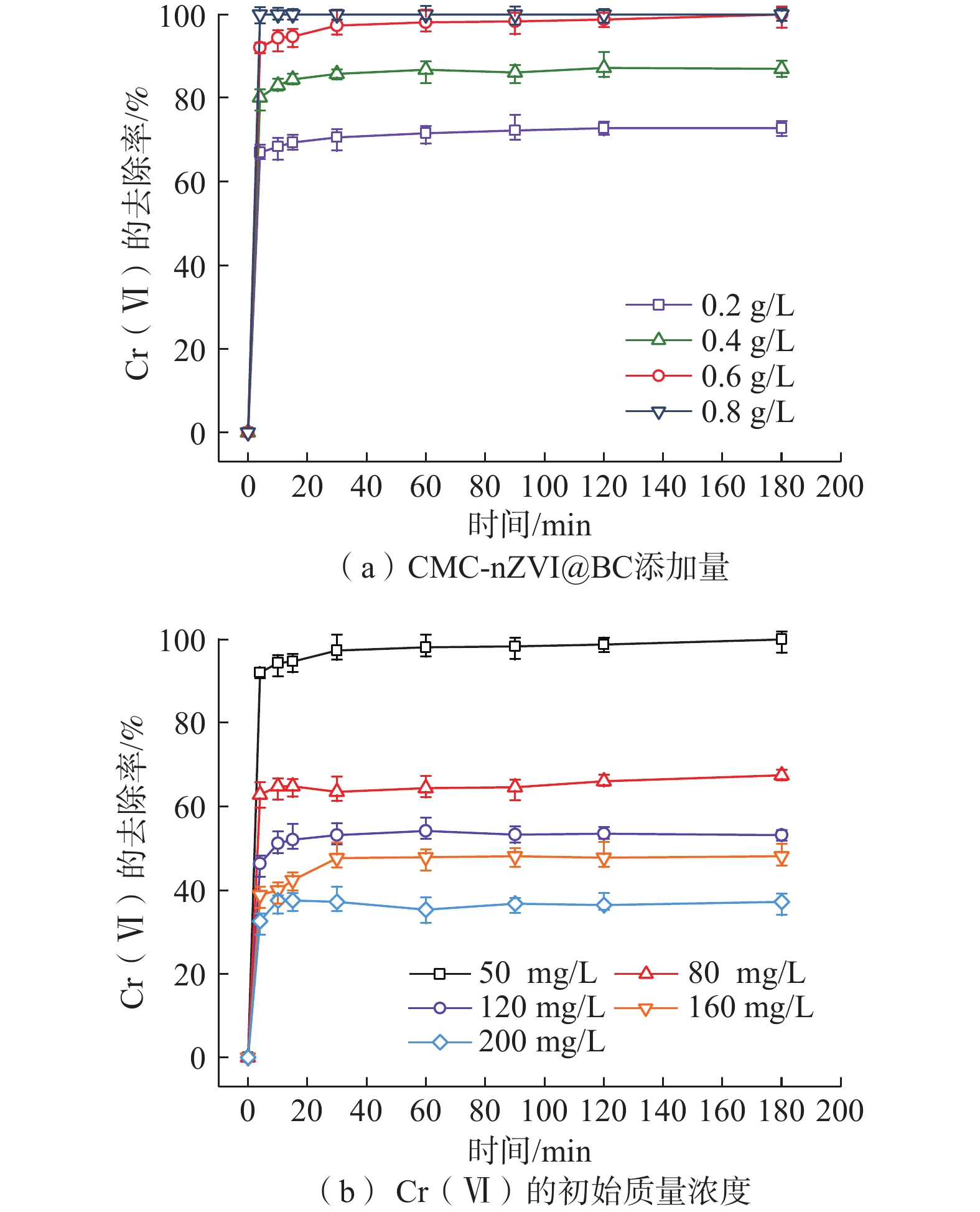
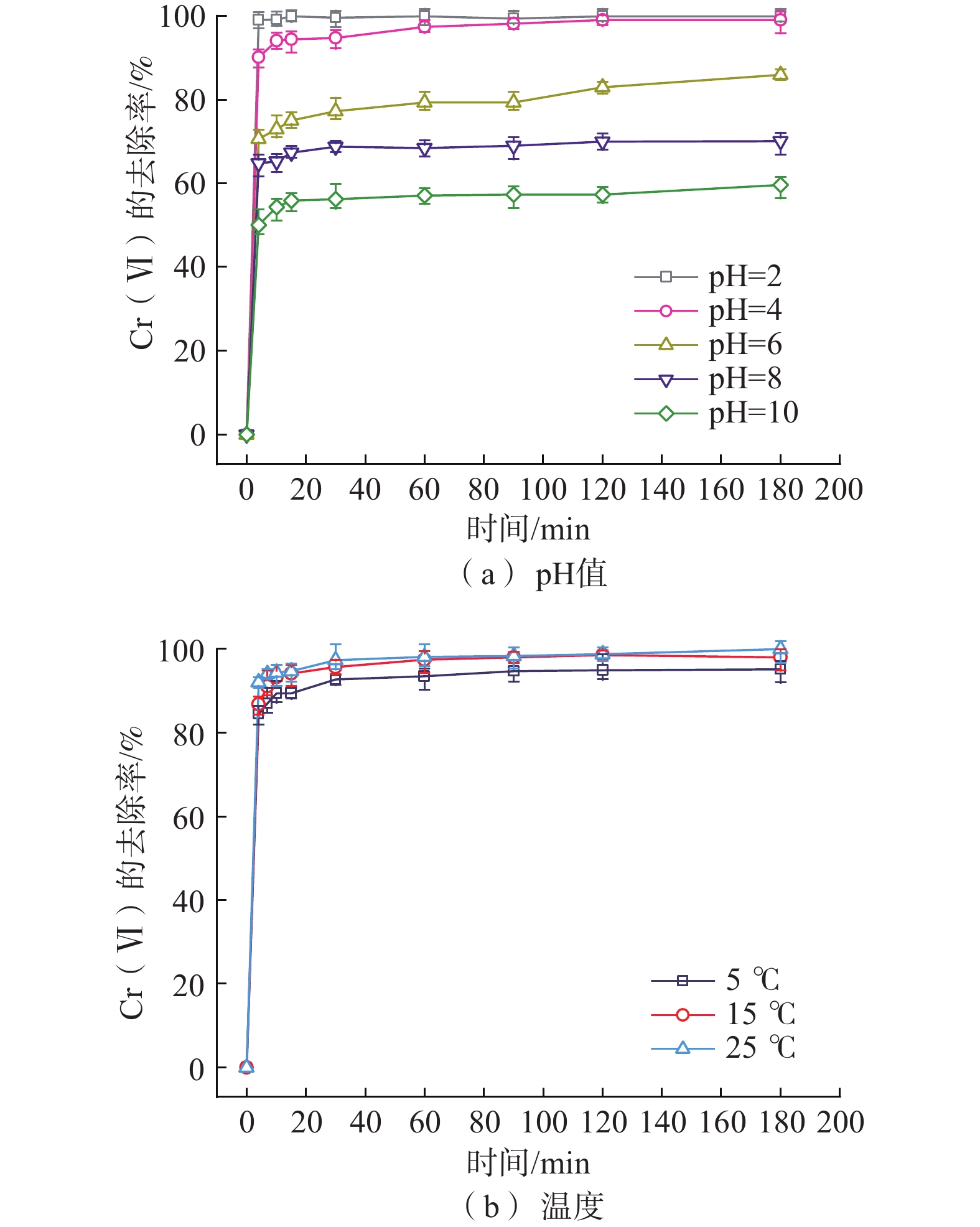
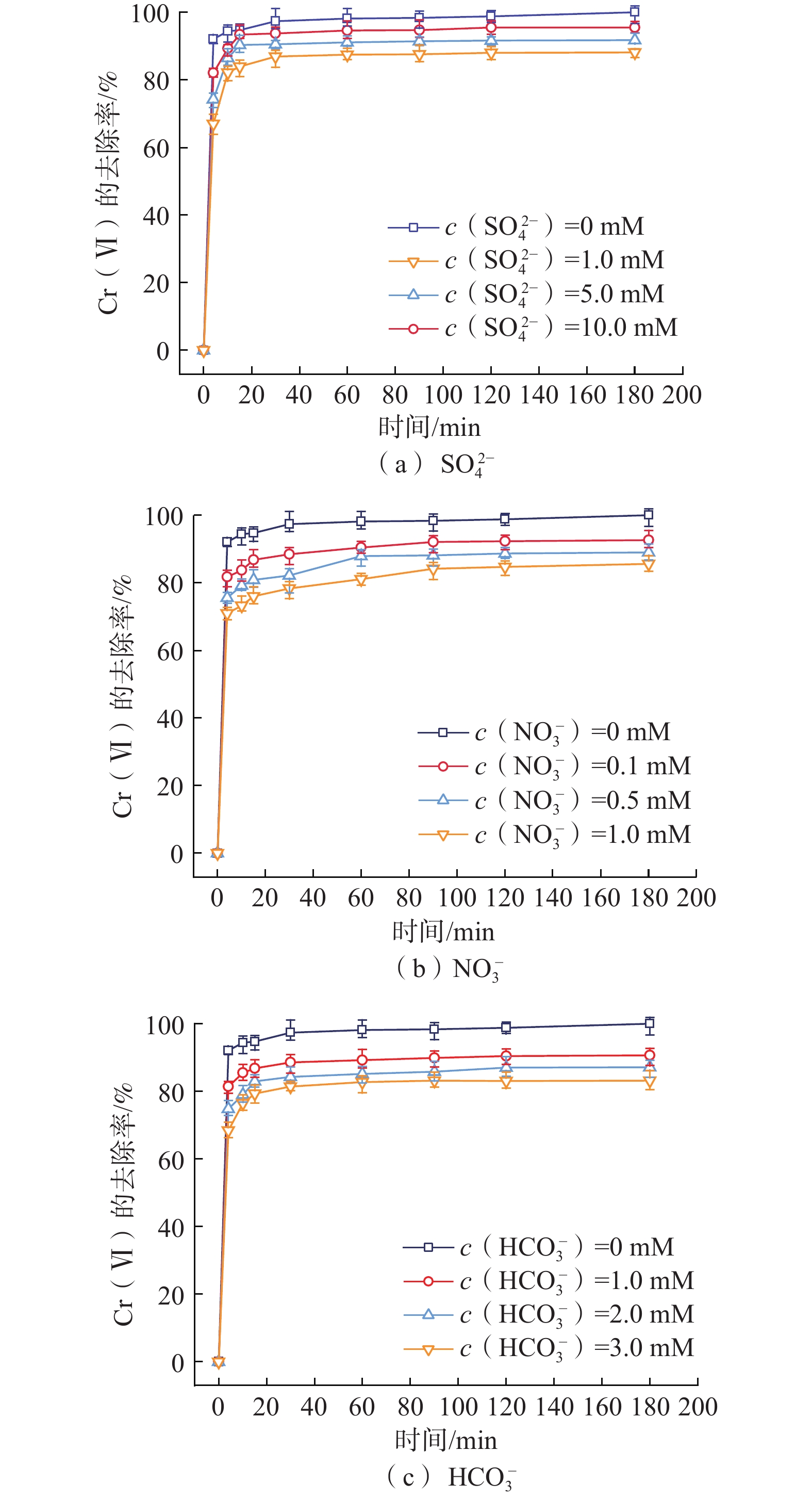
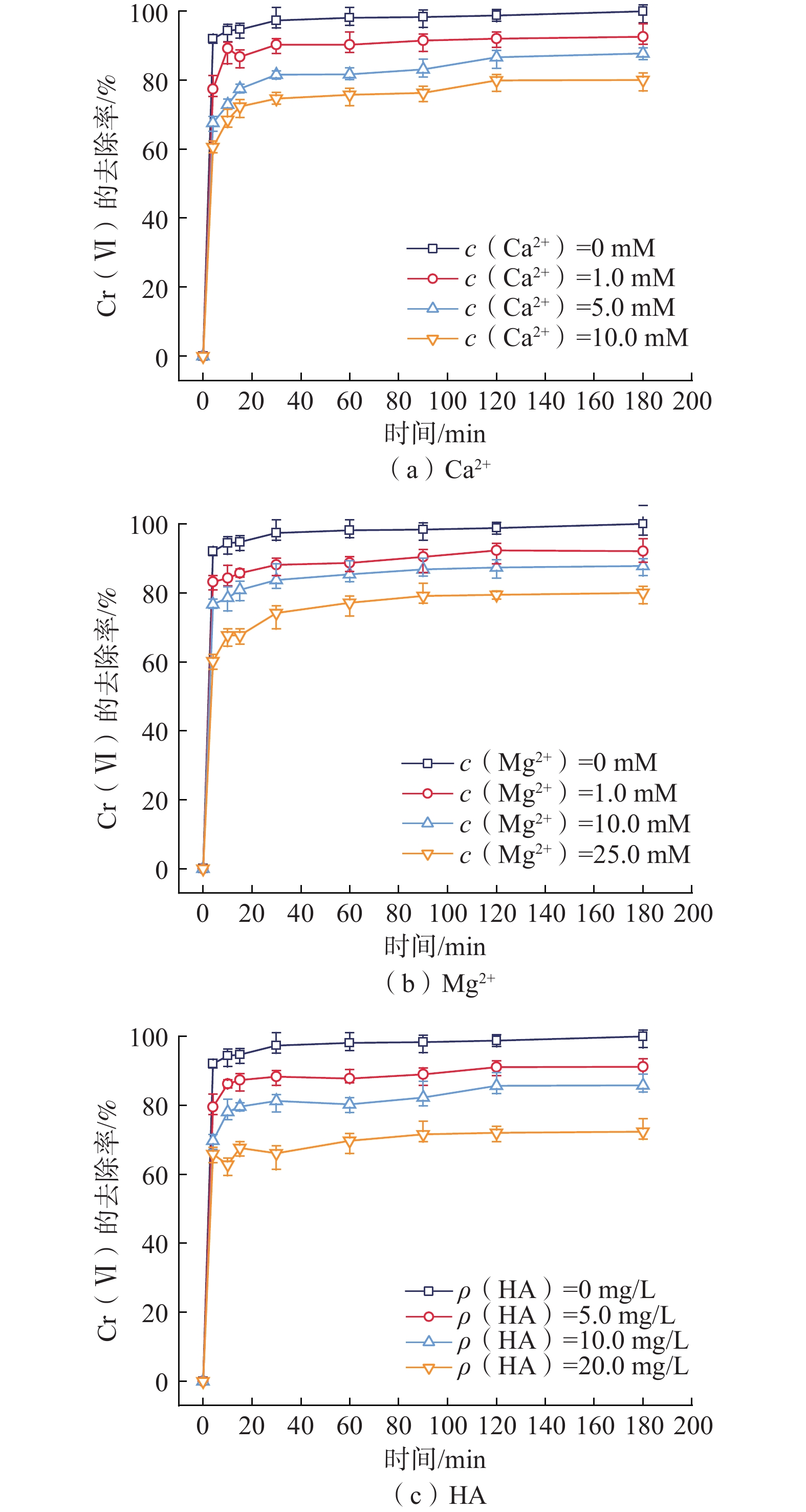
纳米零价铁(nZVI)存在易团聚、钝化和迁移性差等问题,影响对六价铬[Cr(VI)]污染地下水的原位修复效果。为了开发一种低成本、绿色的nZVI改性材料,以球磨生物炭(BC)为载体负载nZVI,构建了nZVI@BC反应体系,再利用羧甲基纤维素(CMC)稳定nZVI@BC,合成了一种新型高效、抗钝化纳米级别的修复材料CMC-nZVI@BC。对改性前后的nZVI进行表征分析,探究了材料添加量、Cr(VI)初始质量浓度、初始pH值、温度及地下水化学组分对CMC-nZVI@BC去除Cr(VI)的影响,并阐明去除Cr(VI)的机理。得出如下结论:(1)铁碳质量比为2∶1时的nZVI@BC对Cr(VI)的去除效果最好, 3 h内0.6 g/L CMC-nZVI@BC对50 mg/L Cr(VI)的去除率达99.9%,表现出较高的去除Cr(VI)的速率和能力;(2)去除Cr(VI)的主要机制是通过还原和沉淀反应;(3)在pH值2~10范围内,pH值对去除Cr(VI)有显著影响,温度影响较小;(4)${\mathrm{SO}}_4^{2-}$的存在促进了Cr(VI)的去除,而${\mathrm{HCO}}_3^{-} $、${\mathrm{NO}}_3^{-} $、Ca2+、Mg2+和腐殖酸对Cr(VI)的去除均有不同程度的抑制作用。这些结果表明,CMC-nZVI@BC可以作为有效去除Cr(VI)的原位修复药剂,为nZVI在地下水原位修复的应用提供了依据。
Abstract:Nano zero-valent iron (nZVI) has problems such as agglomeration, passivation and poor transportability, which affect the in situ remediation effect of Cr(VI) contaminated groundwater. To develop a low-cost, green nZVI modified material, a low-cost, green modification for nZVI was developed. The nZVI@BC reaction system was constructed by supporting nZVI with ball-milled biochar (BC) as carrier and then stabilized with carboxymethyl cellulose (CMC). CMC-nZVI@BC was synthesized as a novel high-efficiency, anti-passivation nano-scale remediation material. The nZVI before and after modification was characterized and analyzed, and the effects of CMC-nZVI@BC addition, initial concentration of Cr(VI), pH and temperature and chemical fraction of groundwater on the removal of Cr(VI) by CMC-nZVI@BC were investigated, and the mechanism of Cr(VI) removal by CMC-nZVI@BC was elucidated, and the following conclusions were obtained: (1) The best removal of Cr(VI) by nZVI@BC at the Fe and C mass ratio of 2∶1; the removal rate of 50 mg/L Cr(VI) by 0.6 g/L CMC-nZVI@BC within 3 h reached 99.9%, exhibiting a high removal rate and capacity of Cr(VI). (2) The main mechanism of Cr(VI) removal by CMC-nZVI@BC was reduction and precipitation. (3) In the range of 2 to 10, the pH value had a significant effect on the removal of Cr(VI) by CMC-nZVI@BC, with less effect of temperature. (4) The presence of ${\mathrm{SO}}_4^{2-} $ promoted Cr(VI) removal, while ${\mathrm{HCO}}_3^{-} $, ${\mathrm{NO}}_3^{-} $, Ca2+, Mg2+ and humic acid, all had different degrees of inhibition on Cr(VI) removal. These results suggest that CMC-nZVI@BC can be an effective in situ remediation agent for Cr(VI) removal, offering the possibility of applying nZVI for in situ groundwater remediation.
-
Key words:
- groundwater remediation /
- nano zero-valent iron /
- biochar /
- carboxymethyl cellulose /
- Cr(VI)
-

-
表 1 反应体系中离子浓度
Table 1. Ion concentration in the reaction system
离子类型 浓度梯度 单位 Cl− 0,1.0,5.0,10.0 mM ${\mathrm{SO}}_4^{2-} $ 0,1.0,5.0,10.0 mM ${\mathrm{NO}}_3^{-} $ 0,0.1,0.5,1.0 mM ${\mathrm{HCO}}_3^{-} $ 0,1.0,2.0,3.0 mM Ca2+ 0,1.0,5.0,10.0 mM Mg2+ 0,1.0,10.0,25.0 mM HA 0,5.0,10.0,20.0 mg·L−1 表 2 各材料的中位径、比表面积、孔径和孔体积
Table 2. Particle diameter, Specific surface area, pore size and pore volume of materials
材料 中位径/
nm比表面积/
(m2·g−1)孔径/
nm孔体积/
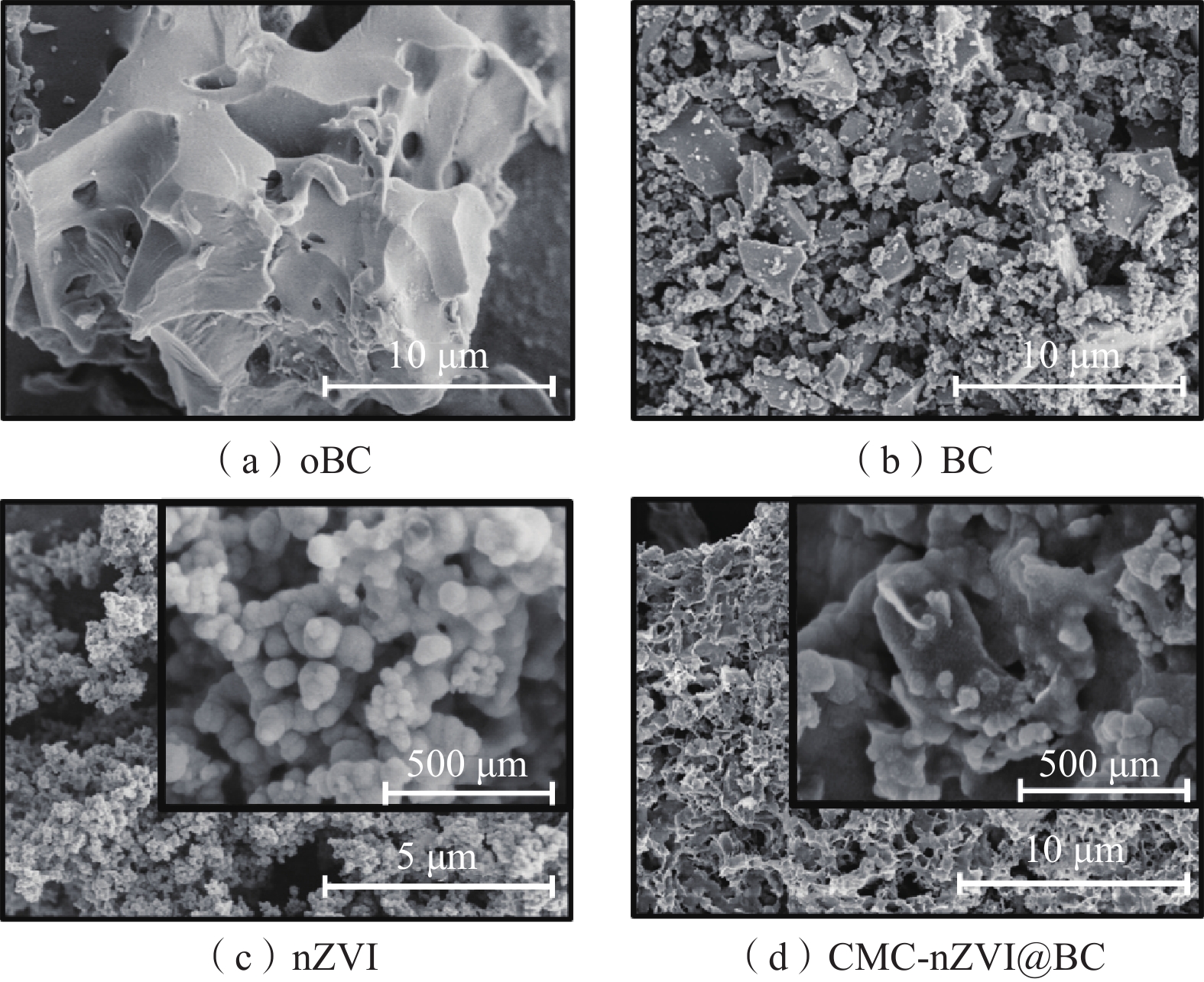
(cm3·g−1)oBC — 89.9313 2.3517 0.0527 BC 384.7 363.1087 2.7838 0.2527 nZVI 329.1 13.8399 9.9702 0.0245 nZVI@BC 463.6 198.8211 5.9234 0.2944 CMC-nZVI@BC 420.1 37.8883 14.2939 0.1354 注:表中“—”为未检测。 -
[1] 徐迎春,杨丽虎,宋献方,等. 基于保护敏感目标的场地地下水污染风险评估[J]. 地质科技通报,2023,42(3):262 − 271. [XU Yingchun,YANG Lihu,SONG Xianfang,et al. Site groundwater pollution risk assessment based on the protection of sensitive receptors[J]. Bulletin of Geological Science and Technology,2023,42(3):262 − 271. (in Chinese with English abstract)
XU Yingchun, YANG Lihu, SONG Xianfang, et al . Site groundwater pollution risk assessment based on the protection of sensitive receptors[J]. Bulletin of Geological Science and Technology,2023 ,42 (3 ):262 −271 . (in Chinese with English abstract)[2] TROIANO J M,JORDAN D S,HULL C J,et al. Interaction of Cr(Ⅲ) and Cr(VI) with hematite studied by second harmonic generation[J]. The Journal of Physical Chemistry C,2013,117(10):5164 − 5171. doi: 10.1021/jp3122819
[3] JOBBY R,JHA P,YADAV A K,et al. Biosorption and biotransformation of hexavalent chromium[Cr(VI)]:A comprehensive review[J]. Chemosphere,2018,207:255 − 266. doi: 10.1016/j.chemosphere.2018.05.050
[4] CRANE R A,SCOTT T B. Nanoscale zero-valent iron:Future prospects for an emerging water treatment technology[J]. Journal of Hazardous Materials,2012,211/212:112 − 125. doi: 10.1016/j.jhazmat.2011.11.073
[5] XIE Jituo,LEI Chao,CHEN Wenqian,et al. Catalytic properties of transition metals modified nanoscale zero-valent iron for simultaneous removal of 4-chlorophenol and Cr(VI):Efficacy,descriptor and reductive mechanisms[J]. Journal of Hazardous Materials,2021,403:123827. doi: 10.1016/j.jhazmat.2020.123827
[6] LI Dan,MAO Zhe,ZHONG Yin,et al. Reductive transformation of tetrabromobisphenol A by sulfidated nano zerovalent iron[J]. Water Research,2016,103:1 − 9. doi: 10.1016/j.watres.2016.07.003
[7] WEI Yuzhen,USMAN M,FAROOQ M,et al. Removing hexavalent chromium by nano zero-valent iron loaded on attapulgite[J]. Water,Air,& Soil Pollution,2022,233(2):1 − 14.
[8] LI Yaru,ZHAO Heping,ZHU Lizhong. Remediation of soil contaminated with organic compounds by nanoscale zero-valent iron:A review[J]. Science of the Total Environment,2021,760:143413. doi: 10.1016/j.scitotenv.2020.143413
[9] WANG Shengsen,ZHAO Mingyue,ZHOU Min,et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water:A critical review[J]. Journal of Hazardous Materials,2019,373:820 − 834. doi: 10.1016/j.jhazmat.2019.03.080
[10] LIANG Weiyu,WANG Gehui,PENG Cheng,et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water:A critical review[J]. Journal of Hazardous Materials,2022,426:127993. doi: 10.1016/j.jhazmat.2021.127993
[11] KLÜPFEL L,KEILUWEIT M,KLEBER M,et al. Redox properties of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology,2014,48(10):5601 − 5611.
[12] 张建,马锋锋,郝爱红,等. 改性生物炭对水中Cr(Ⅵ)的去除研究进展[J]. 环境科学与技术,2020,43(12):38 − 46. [ZHANG Jian,MA Fengfeng,HAO Aihong,et al. Research progress of Cr(Ⅵ) removal from water by modified biochar[J]. Environmental Science & Technology,2020,43(12):38 − 46. (in Chinese with English abstract) doi: 10.19672/j.cnki.1003-6504.2020.12.006
ZHANG Jian, MA Fengfeng, HAO Aihong, et al . Research progress of Cr(Ⅵ) removal from water by modified biochar[J]. Environmental Science & Technology,2020 ,43 (12 ):38 −46 . (in Chinese with English abstract)[13] SEMERÁD J,ŠEVCŮ A,NGUYEN N H A,et al. Discovering the potential of an nZVI-biochar composite as a material for the nanobioremediation of chlorinated solvents in groundwater:Degradation efficiency and effect on resident microorganisms[J]. Chemosphere,2021,281:130915. doi: 10.1016/j.chemosphere.2021.130915
[14] LYU Honghong,GAO Bin,HE Feng,et al. Effects of ball milling on the physicochemical and sorptive properties of biochar:Experimental observations and governing mechanisms[J]. Environmental Pollution,2018,233:54 − 63. doi: 10.1016/j.envpol.2017.10.037
[15] SHAN Danna,DENG Shubo,ZHAO Tianning,et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials,2016,305:156 − 163. doi: 10.1016/j.jhazmat.2015.11.047
[16] ZHAO Xiao,LIU Wen,CAI Zhengqing,et al. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation[J]. Water Research,2016,100:245 − 266. doi: 10.1016/j.watres.2016.05.019
[17] LI Tielong,GAO Chaolin,WANG Wei,et al. Strong influence of degree of substitution on carboxymethyl cellulose stabilized sulfidated nanoscale zero-valent iron[J]. Journal of Hazardous Materials,2022,425:128057. doi: 10.1016/j.jhazmat.2021.128057
[18] MURAD H A,AHMAD M,BUNDSCHUH J,et al. A remediation approach to chromium-contaminated water and soil using engineered biochar derived from peanut shell[J]. Environmental Research,2022,204:112125. doi: 10.1016/j.envres.2021.112125
[19] SUN Yuankui,LI Jinxiang,HUANG Tinglin,et al. The influences of iron characteristics,operating conditions and solution chemistry on contaminants removal by zero-valent iron:A review[J]. Water Research,2016,100:277 − 295. doi: 10.1016/j.watres.2016.05.031
[20] 国家质量监督检验检疫总局,中国国家标准化管理委员会. 地下水质量标准:GB/T 14848—2017[S]. 北京:中国标准出版社,2017. [General Administration of Quality Supervision,Inspection and Quarantine of the People’s Republic of China,Standardization Administration of the People’s Republic of China. Standard for groundwater quality:GB/T 14848—2017[S]. Beijing:Standards Press of China,2017. (in Chinese)
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Standard for groundwater quality: GB/T 14848—2017[S]. Beijing: Standards Press of China, 2017. (in Chinese) [21] ZHU Fang,HE Siying,LIU Tao. Effect of pH,temperature and co-existing anions on the Removal of Cr(VI) in groundwater by green synthesized nZVI/Ni[J]. Ecotoxicology and Environmental Safety,2018,163:544 − 550. doi: 10.1016/j.ecoenv.2018.07.082
[22] 国家环境保护局. 水质 六价铬的测定 二苯碳酰二肼分光光度法:GB 7467—1987[S]. 北京:中国标准出版社,1987. [State Bureau of Environmental Protection of the People’s Republic of China. Water quality-determination of chromium(6)- 1.5 diphenylcarbahydrazide spectrophotometric method:GB 7467—1987[S]. Beijing:Standards Press of China,1987. (in Chinese)
State Bureau of Environmental Protection of the People’s Republic of China. Water quality-determination of chromium(6)- 1.5 diphenylcarbahydrazide spectrophotometric method: GB 7467—1987[S]. Beijing: Standards Press of China, 1987. (in Chinese) [23] 中华人民共和国国家环境保护总局. 水质 铁的测定 邻菲啰啉分光光度法(试行):HJ/T 345—2007[S]. 北京:中国环境科学出版社,2007. [State Environmental Protection Administration of the People’s Republic of China. Water quality-determination of iron-phenanthroline spectrophotometry:HJ/T 345—2007[S]. Beijing:China Environmental Science Press,2007. (in Chinese)
State Environmental Protection Administration of the People’s Republic of China. Water quality-determination of iron-phenanthroline spectrophotometry: HJ/T 345—2007[S]. Beijing: China Environmental Science Press, 2007. (in Chinese) [24] CHEN Zhongshan,WEI Dongli,LI Qian,et al. Macroscopic and microscopic investigation of Cr(VI) immobilization by nanoscaled zero-valent iron supported zeolite MCM-41 via batch,visual,XPS and EXAFS techniques[J]. Journal of Cleaner Production,2018,181:745 − 752. doi: 10.1016/j.jclepro.2018.01.231
[25] DU Jiangkun,BAO Jianguo,LU Chenghang,et al. Reductive sequestration of chromate by hierarchical FeS@Fe0 particles[J]. Water Research,2016,102:73 − 81. doi: 10.1016/j.watres.2016.06.009
[26] 赵玲子. CMC改性硫化纳米零价铁原位反应带修复Cr(Ⅵ)污染地下水研究[D]. 长春:吉林大学,2020. [ZHAO Lingzi. Study on the remediation of hexavalent chromium contaminated groundwater with in-situ reaction zone of carboxymethyl cellulose modified sulfidated nano zerovalent iron[D]. Changchun:Jilin University,2020. (in Chinese with English abstract)
ZHAO Lingzi. Study on the remediation of hexavalent chromium contaminated groundwater with in-situ reaction zone of carboxymethyl cellulose modified sulfidated nano zerovalent iron[D]. Changchun: Jilin University, 2020. (in Chinese with English abstract) [27] SHI Weilin,SONG Xue. Removal of hexavalent chromium from aqueous using biochar supported nanoscale zero-velent iron[M]//Springer Proceedings in Energy. Singapore:Springer Singapore,2018:885 − 895.
[28] DENG Shihai,LI Desheng,YANG Xue,et al. Iron[Fe(0)]-rich substrate based on iron-carbon micro-electrolysis for phosphorus adsorption in aqueous solutions[J]. Chemosphere,2017,168:1486 − 1493. doi: 10.1016/j.chemosphere.2016.11.043
[29] WANG Xiao,ZHANG Yue,WANG Zhiwei,et al. Advances in metal (loid) oxyanion removal by zerovalent iron:Kinetics,pathways,and mechanisms[J]. Chemosphere,2021,280:130766. doi: 10.1016/j.chemosphere.2021.130766
[30] LIU Wanting,BAI Jing,CHI Zifang,et al. An in situ reactive zone with xanthan gum modified reduced graphene oxide supported nanoscale zero-valent iron (XG-nZVI/rGO) for remediation of Cr(VI)-polluted aquifer:Dynamic evolutions of Cr(VI) and environmental variables[J]. Journal of Environmental Chemical Engineering,2021,9(1):104987. doi: 10.1016/j.jece.2020.104987
[31] LUKMAN S. Study on integrated electrokinetics-adsorption remediation technique for simultaneous removal of heavy metals and organics from saline-sodic soil:Eeffects of operating parameters[D]. University of Hafr Al-Batin,2013.
[32] QIU Yue,ZHANG Qian,GAO Bin,et al. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron:Synergy of adsorption,reduction and transformation[J]. Environmental Pollution,2020,265:115018. doi: 10.1016/j.envpol.2020.115018
[33] 侯素珍,田浩然,黄超,等. 氨基改性生物炭负载纳米零价铁去除水中Cr(Ⅵ)[J]. 环境科学学报,2020,40(11):3931 − 3938. [HOU Suzhen,TIAN Haoran,HUANG Chao,et al. Removal of Cr(Ⅵ) from aqueous solution by amino-modified biochar supported nano zero-valent iron[J]. Acta Scientiae Circumstantiae,2020,40(11):3931 − 3938. (in Chinese with English abstract)
HOU Suzhen, TIAN Haoran, HUANG Chao, et al . Removal of Cr(Ⅵ) from aqueous solution by amino-modified biochar supported nano zero-valent iron[J]. Acta Scientiae Circumstantiae,2020 ,40 (11 ):3931 −3938 . (in Chinese with English abstract)[34] TANDON R K,CRISP P T,ELLIS J,et al. Effect of pH on chromium(VI) species in solution[J]. Talanta,1984,31(3):227 − 228. doi: 10.1016/0039-9140(84)80059-4
[35] TAN Xiangpeng,SHAABAN M,YANG Jianwei,et al. Efficient removal of hexavalent chromium from an aquatic system using nanoscale zero-valent iron supported by ramie biochar[J]. Nanomaterials,2021,11(10):2698. doi: 10.3390/nano11102698
[36] 任黎明. 黄原胶稳定氧化石墨烯负载纳米铁去除地下水中六价铬污染的研究[D]. 长春:吉林大学,2019. [REN Liming. Study on removal of chromium (Ⅵ) polluted groundwater using xanthan gum stabilized graphene oxide-supported nanoscale zero-valent iron[D]. Changchun:Jilin University,2019. (in Chinese with English abstract)
REN Liming. Study on removal of chromium (Ⅵ) polluted groundwater using xanthan gum stabilized graphene oxide-supported nanoscale zero-valent iron[D]. Changchun: Jilin University, 2019. (in Chinese with English abstract) [37] JEONG D,KIM K,MIN D W,et al. Freezing-enhanced dissolution of iron oxides:Effects of inorganic acid anions[J]. Environmental Science & Technology,2015,49(21):12816 − 12822.
[38] RIETRA R P J J,HIEMSTRA T,VAN RIEMSDIJK W H. Electrolyte anion affinity and its effect on oxyanion adsorption on goethite[J]. Journal of Colloid and Interface Science,2000,229(1):199 − 206. doi: 10.1006/jcis.2000.6982
[39] SONG Xiaojie,CHEN Zhihao,WANG Xiaomeng,et al. Ligand effects on nitrate reduction by zero-valent iron:Role of surface complexation[J]. Water Research,2017,114:218 − 227. doi: 10.1016/j.watres.2017.02.040
[40] DEVLIN J F,ALLIN K O. Major anion effects on the kinetics and reactivity of granular iron in glass-encased magnet batch reactor experiments[J]. Environmental Science & Technology,2005,39(6):1868 − 1874.
[41] AHN J Y,KIM C,KIM H S,et al. Effects of oxidants on in situ treatment of a DNAPL source by nanoscale zero-valent iron:A field study[J]. Water Research,2016,107:57 − 65. doi: 10.1016/j.watres.2016.10.037
[42] LU Qiong,JEEN S W,GUI Lai,et al. Nitrate reduction and its effects on trichloroethylene degradation by granular iron[J]. Water Research,2017,112:48 − 57. doi: 10.1016/j.watres.2017.01.031
[43] JEEN S W,GILLHAM R W,BLOWES D W. Effects of carbonate precipitates on long-term performance of granular iron for reductive dechlorination of TCE[J]. Environmental Science & Technology,2006,40(20):6432 − 6437.
[44] PHILLIPS D H,GU B,WATSON D B,et al. Performance evaluation of a zerovalent iron reactive barrier: Mineralogical characteristics[J]. Environmental Science & Technology,2000,34(19):4169 − 4176.
[45] BASNET M,GERSHANOV A,WILKINSON K J,et al. Interaction between palladium-doped zerovalent iron nanoparticles and biofilm in granular porous media:Characterization,transport and viability[J]. Environmental Science:Nano,2016,3(1):127 − 137.
[46] LYU Dan,ZHOU Xiaoxin,ZHOU Jiasheng,et al. Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(II) removal from aqueous solutions[J]. Applied Surface Science,2018,442:114 − 123. doi: 10.1016/j.apsusc.2018.02.085
[47] DONG Haoran,HE Qi,ZENG Guangming,et al. Chromate removal by surface-modified nanoscale zero-valent iron:Effect of different surface coatings and water chemistry[J]. Journal of Colloid and Interface Science,2016,471:7 − 13. doi: 10.1016/j.jcis.2016.03.011
-



 下载:
下载: