Pollution characteristics, migration and transformation of hexavalent chromium in groundwater of a chromium slag
-
摘要:
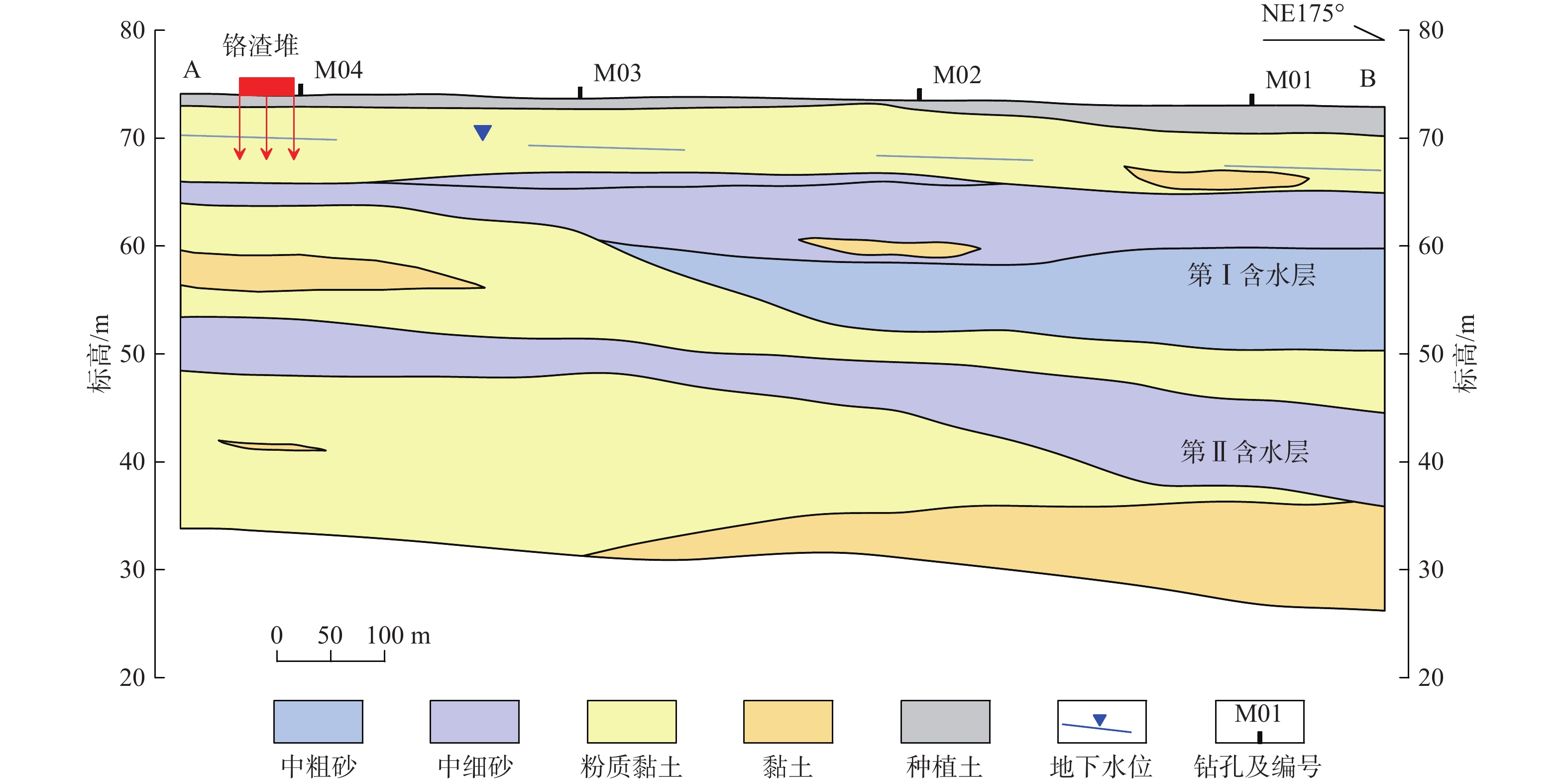
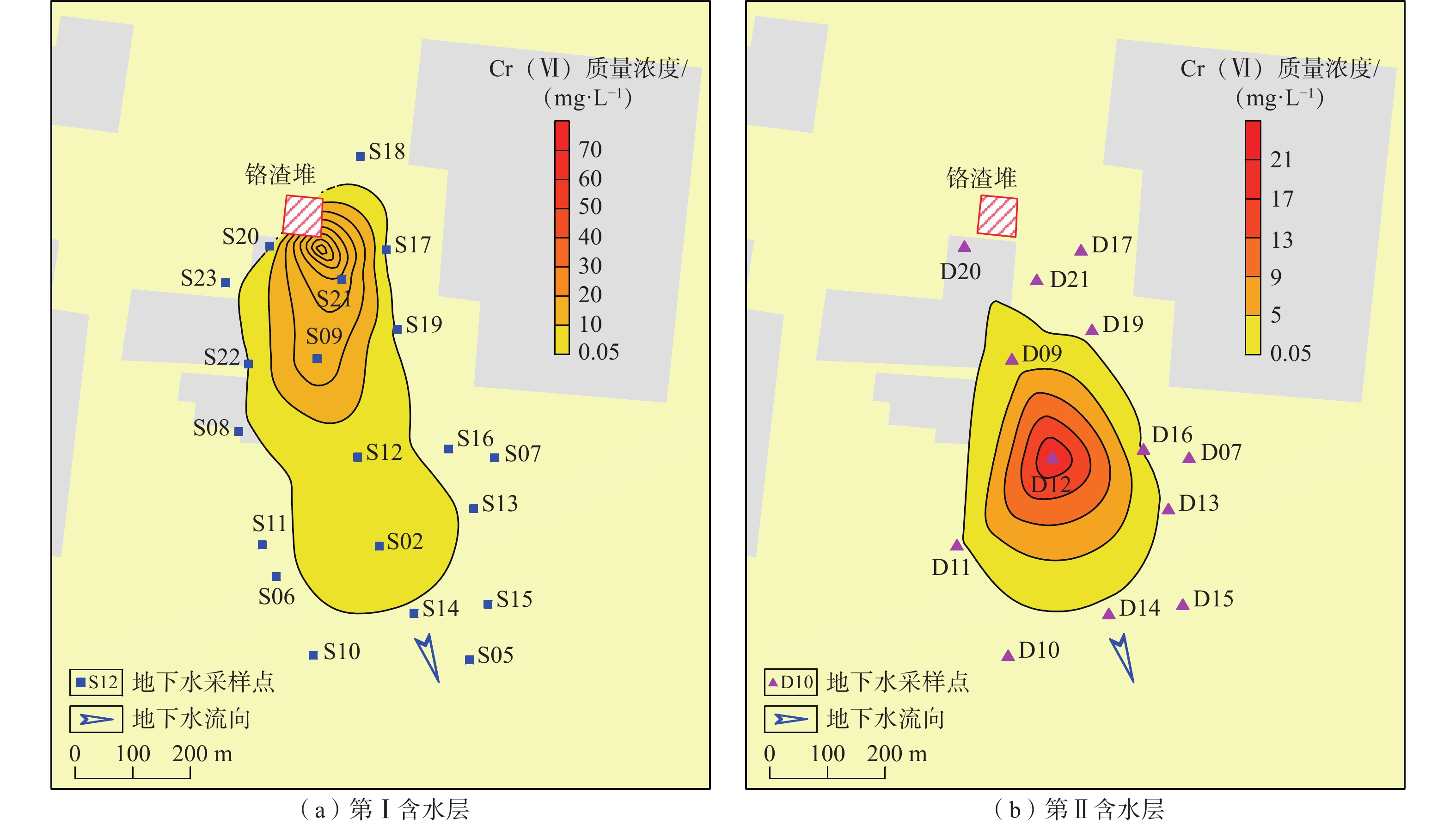
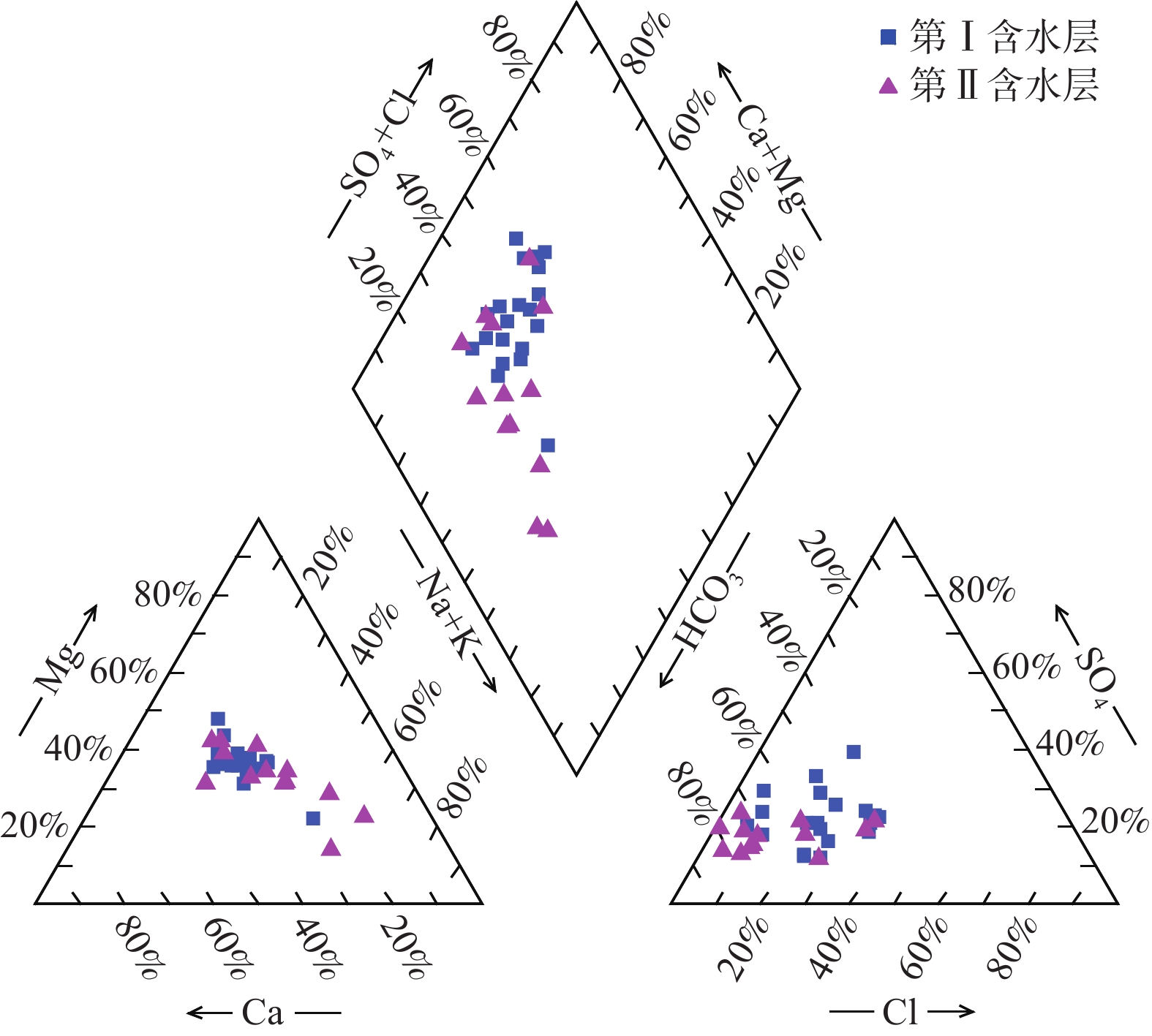
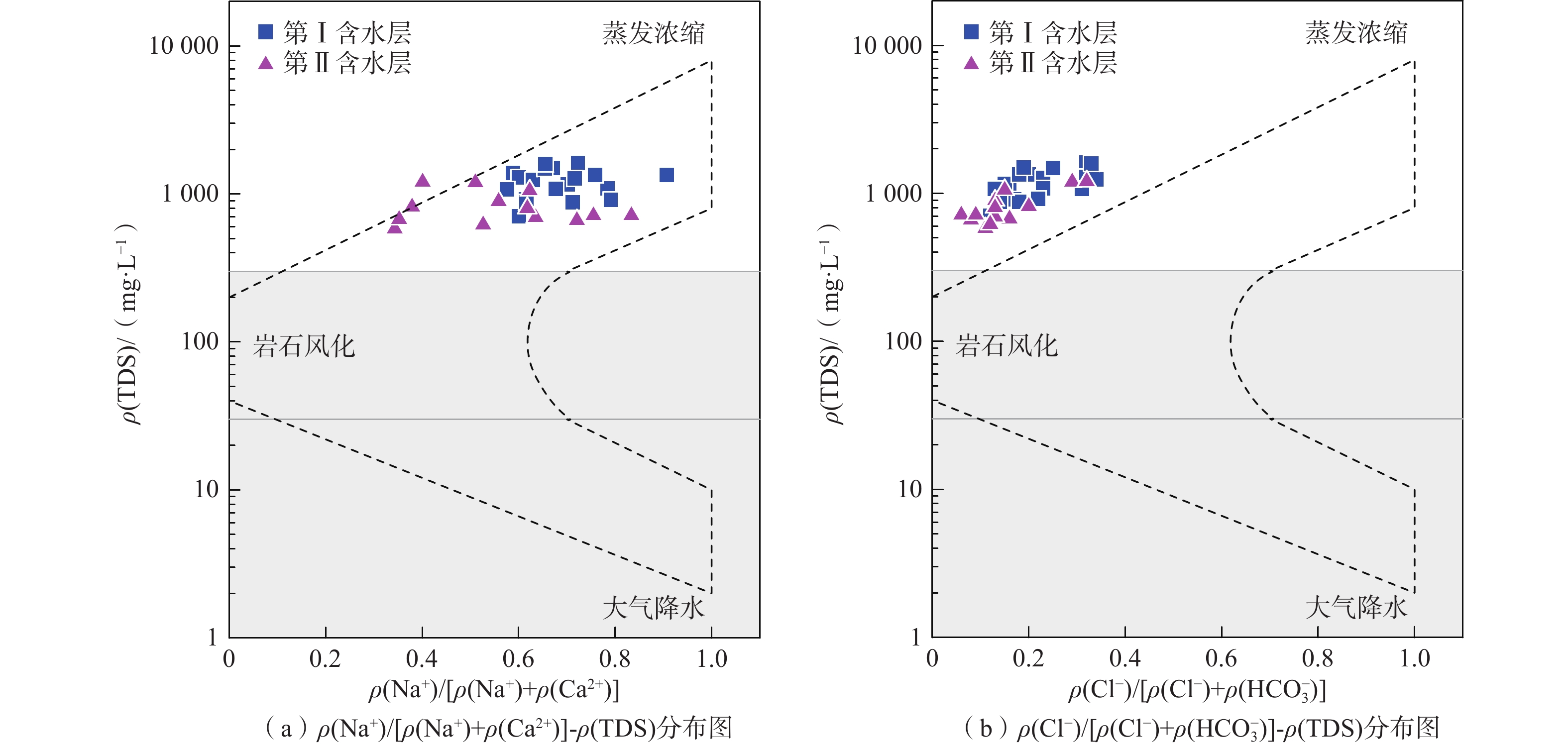
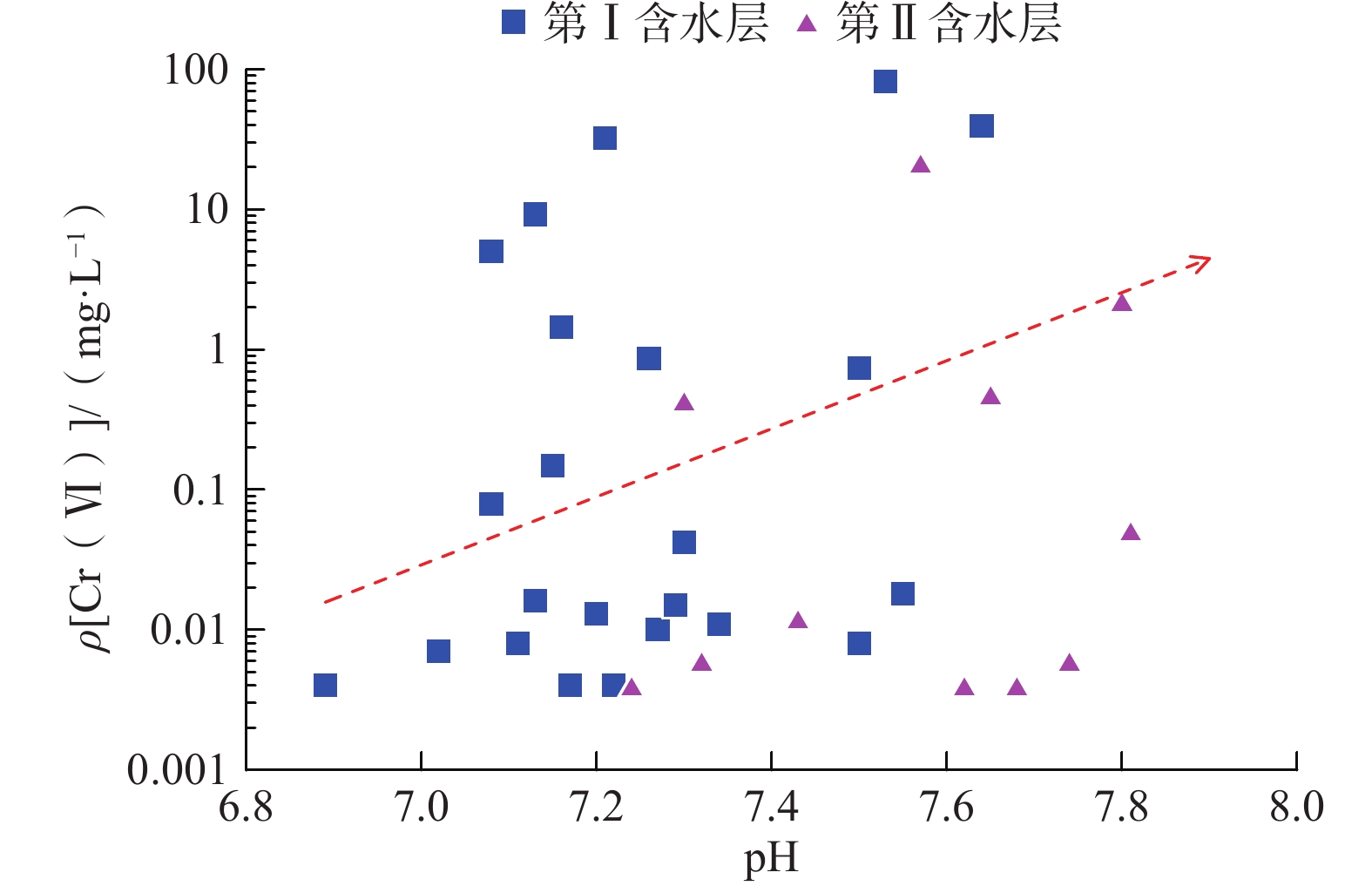
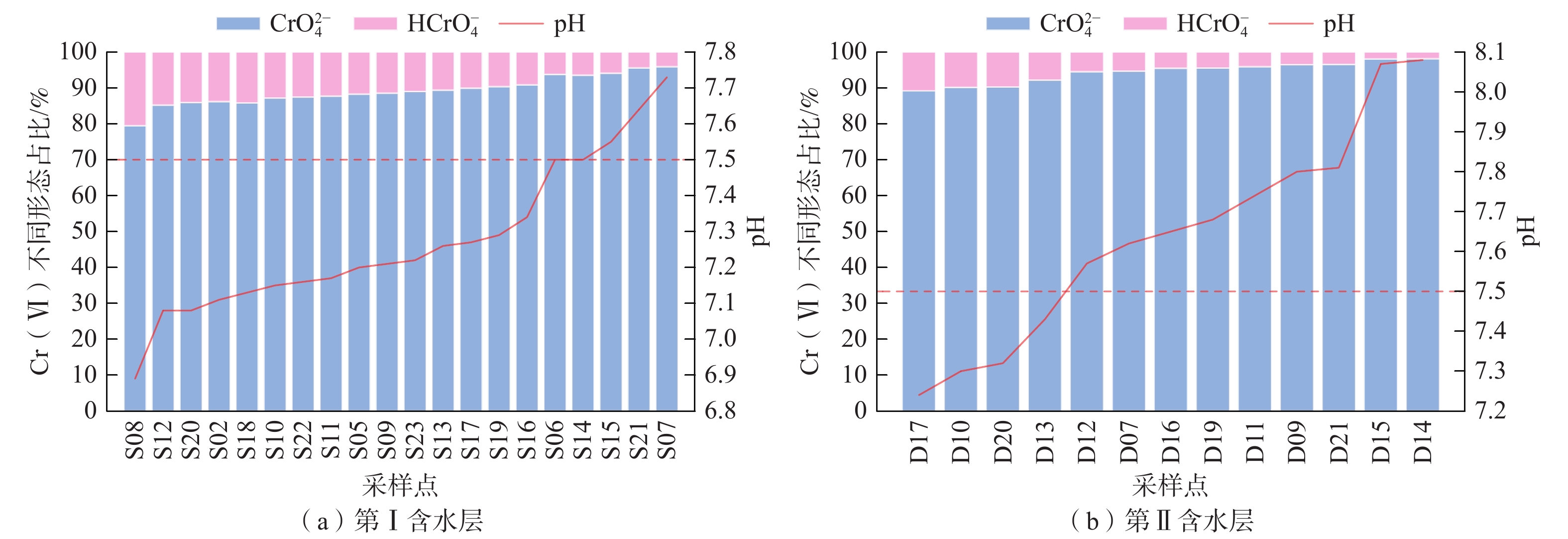
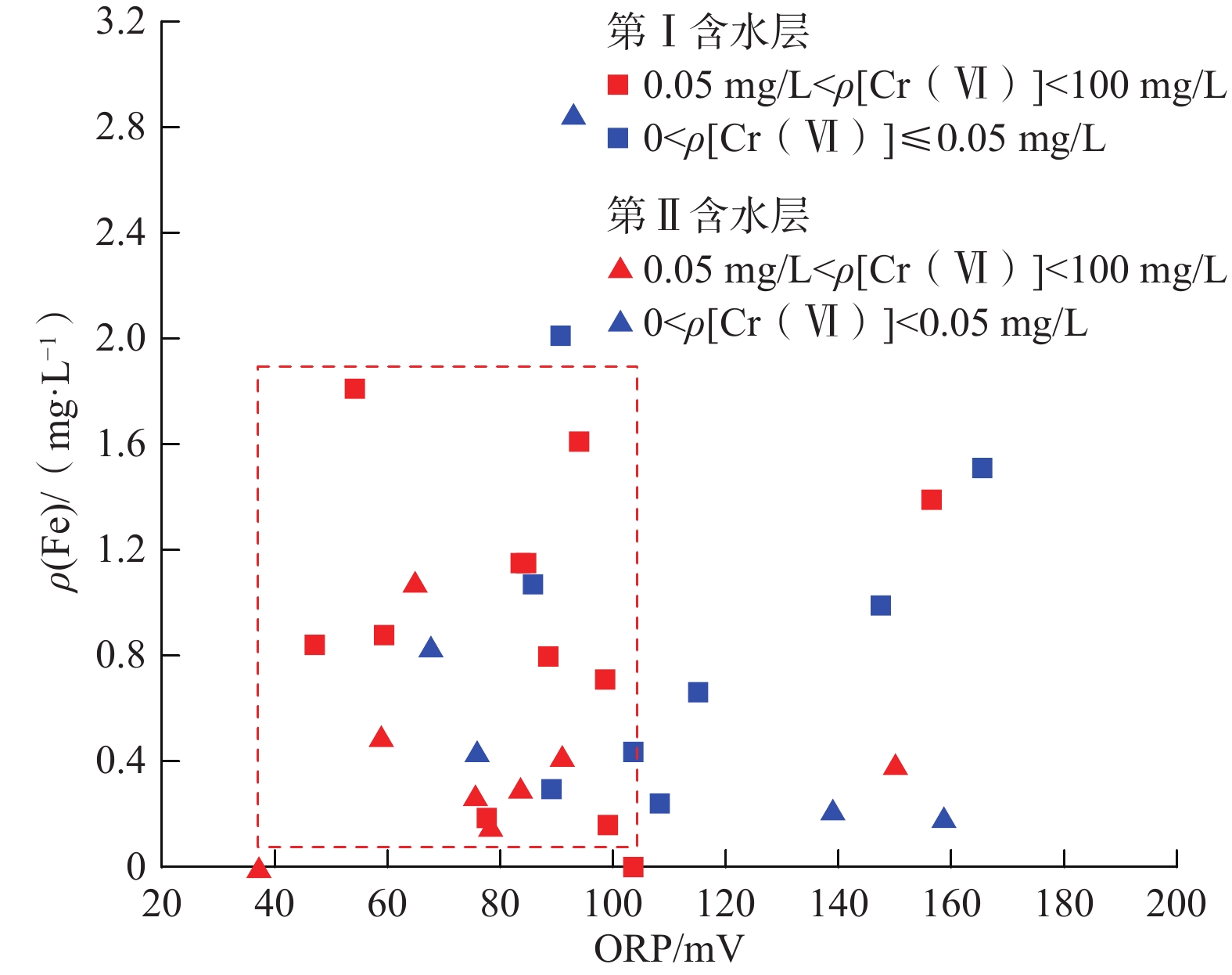
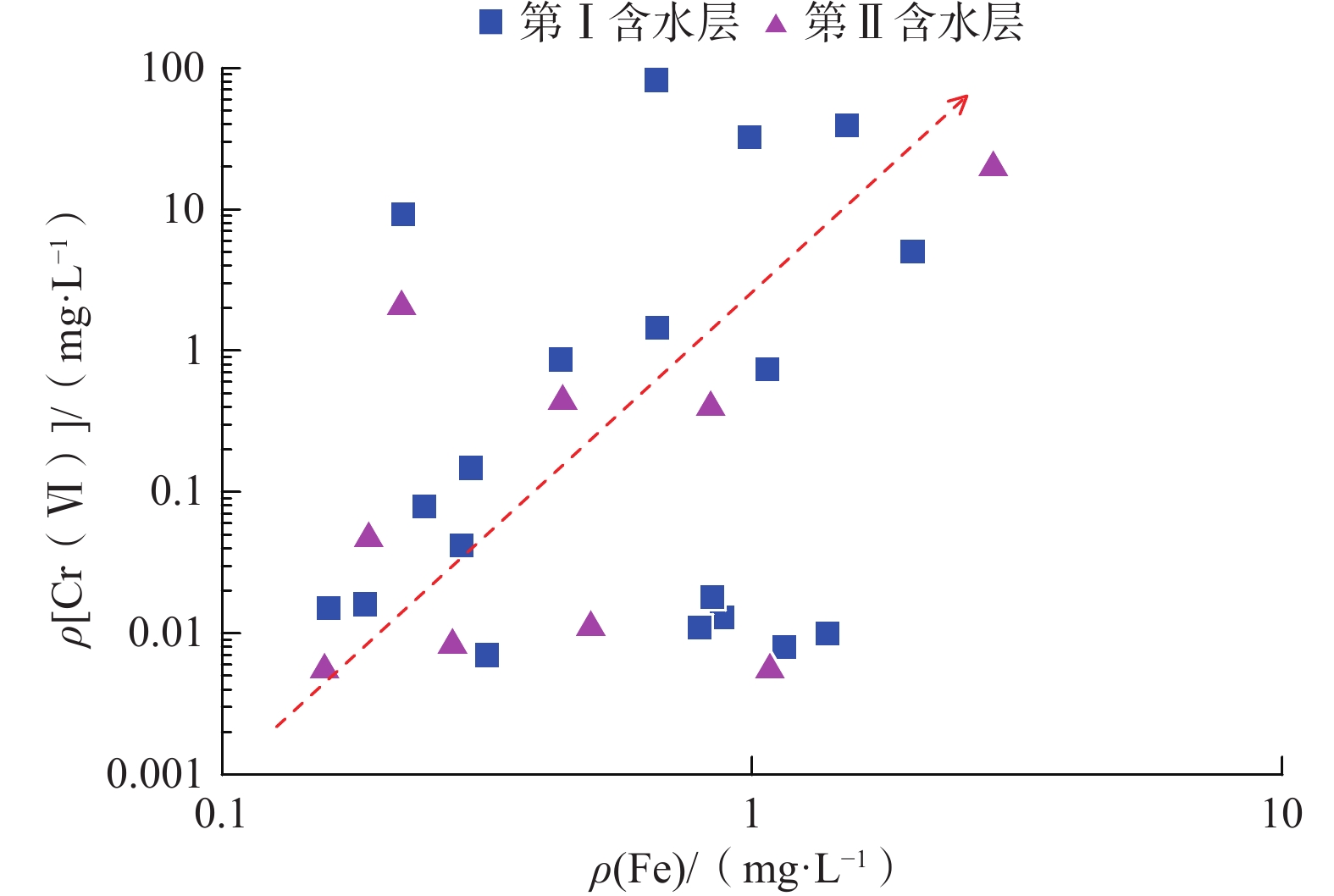
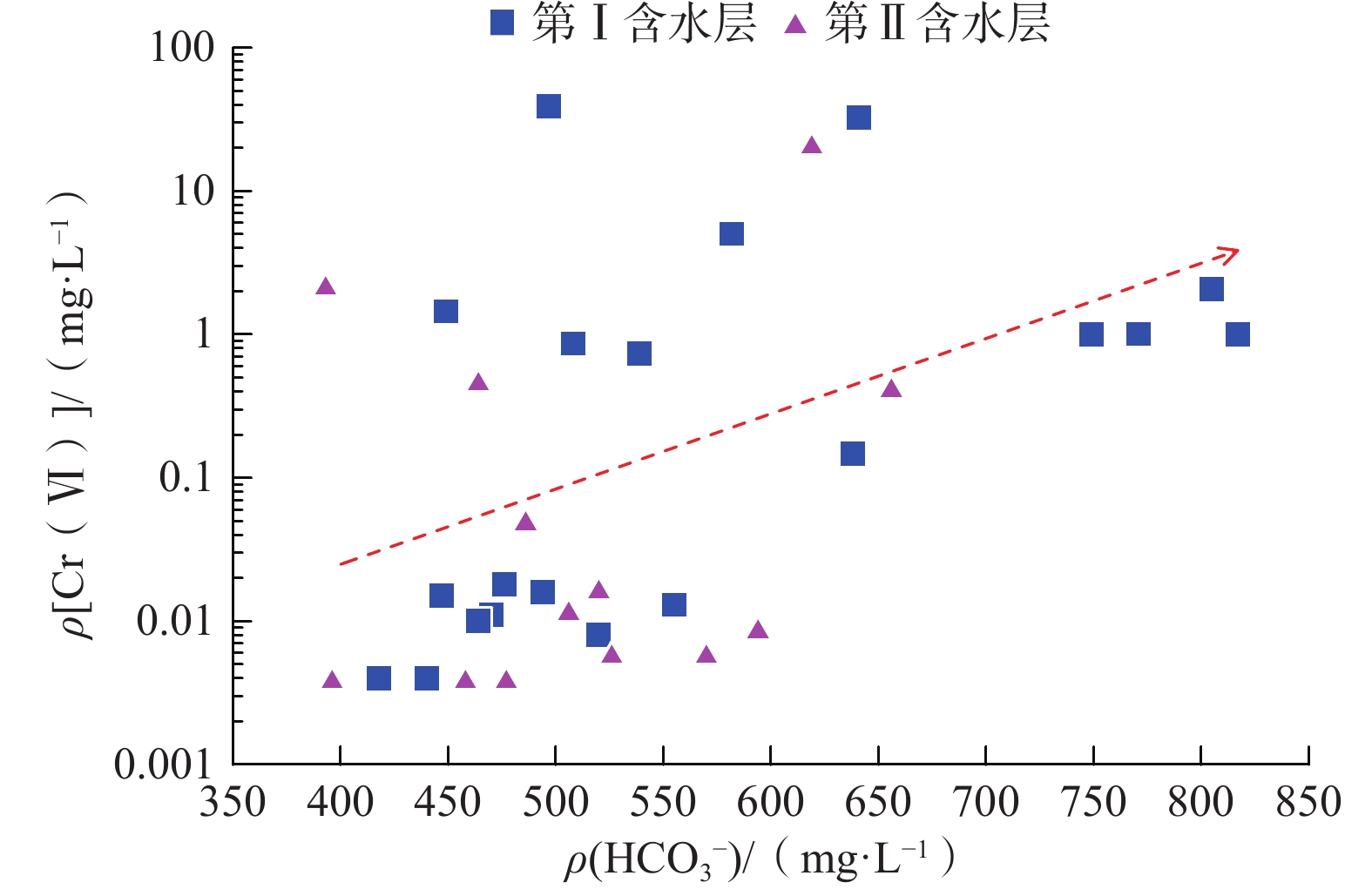
我国铬渣堆历史存量较大,渣堆渗滤液中的六价铬[Cr(Ⅵ)]毒性大、迁移性强。为探究污染源特征、场地水文地质条件和水文地球化学过程综合作用下Cr(Ⅵ)在地下水中的迁移转化规律,文章以某铬污染场地为例,通过水样采集与分析,利用克里格插值、因子分析、水化学计算、Piper三线图和离子比等方法查明地下水中Cr(Ⅵ)的空间分布与水化学特征,识别水体中Cr(Ⅵ)的主要赋存形式,并探讨影响Cr(Ⅵ)在地下水中迁移转化的主控因素。结果表明:(1)场地40 m以浅的 2 个含水层均受到了Cr(Ⅵ)污染,污染范围和程度差异显著。(2)Cr(Ⅵ)在地下水中主要以${\mathrm{CrO}}_4^{2-} $和${\mathrm{HCrO}}_4^- $ 2 种形式存在,${\mathrm{Cr}}_2{\mathrm{O}}_7^{2-} $浓度极低,高浓度Cr(Ⅵ)水点的阴离子以${\mathrm{HCO}}_3^- $和${\mathrm{SO}}_4^{2-} $为主,阳离子以Na+和Ca2+为主。(3)降水淋滤和渗漏导致含有大量Na+、${\mathrm{SO}}_4^{2-} $和Cr(Ⅵ)的渗滤液进入地下水,使地下水pH值升高;高浓度的${\mathrm{HCO}}_3^- $和弱氧化环境下铁氧化物的溶解可以促进Cr(Ⅵ)在地下水中的迁移;锰氧化物和有机质通过氧化还原反应改变地下水中Cr(Ⅵ)浓度;浅层地下水的蒸发浓缩作用加剧Cr(Ⅵ)在地下水中的富集。研究成果可为铬渣类污染场地的风险管控与后期修复提供有力支撑。
Abstract:The historical stockpile of chromium slag in China is large, and the hexavalent chromium in slag leachate is highly toxic and migratory. In order to investigate the migration and transformation pattern of Cr(Ⅵ) in groundwater under the combined effect of pollution source, site hydrogeological condition and hydrogeochemical process, a hexavalent chromium contaminated site was taken as an example in this study, the spatial distribution, hydrogeochemical characteristic, occurrence form and proportion of Cr(Ⅵ) in groundwater, and the main factors affecting migration and transformation of Cr(Ⅵ) are analyzed by sampling and testing groundwater samples, and the combination using of methods such as Kriging interpolation, factor analysis, hydrogeochemical calculation, Piper diagram and ion ratio. The results show that (1) the two aquifers below ground surface 40 m are polluted by Cr(Ⅵ), but the size and degree are different obviously. (2) The main forms of Cr(Ⅵ) in groundwater are ${\mathrm{CrO}}_4^{2-} $ and ${\mathrm{HCrO}}_4^- $, ${\mathrm{Cr}}_2{\mathrm{O}}_7^{2-} $ content is extremely low, and the anions of samples with high Cr(Ⅵ) concentration are mainly ${\mathrm{HCO}}_3^- $ and ${\mathrm{SO}}_4^{2-} $, the cations are mainly Na+ and Ca2+. (3) Precipitation leaching and seepage result in the leachate containing large amounts of Na+, ${\mathrm{SO}}_4^{2-} $ and Cr(Ⅵ) entering groundwater. The increasing pH, high concentrations of ${\mathrm{HCO}}_3^- $ and dissolution of iron oxides under low oxidizing environment in groundwater can facilitate the migration of Cr(Ⅵ). Manganese oxides and organic matter are able to change Cr(Ⅵ) content through redox reaction. Evaporation also plays an important role on the enrichment of Cr(Ⅵ) in groundwater. The results of this research can provide strong support for risk management and post remediation of chromium slag contaminated sites.
-

-
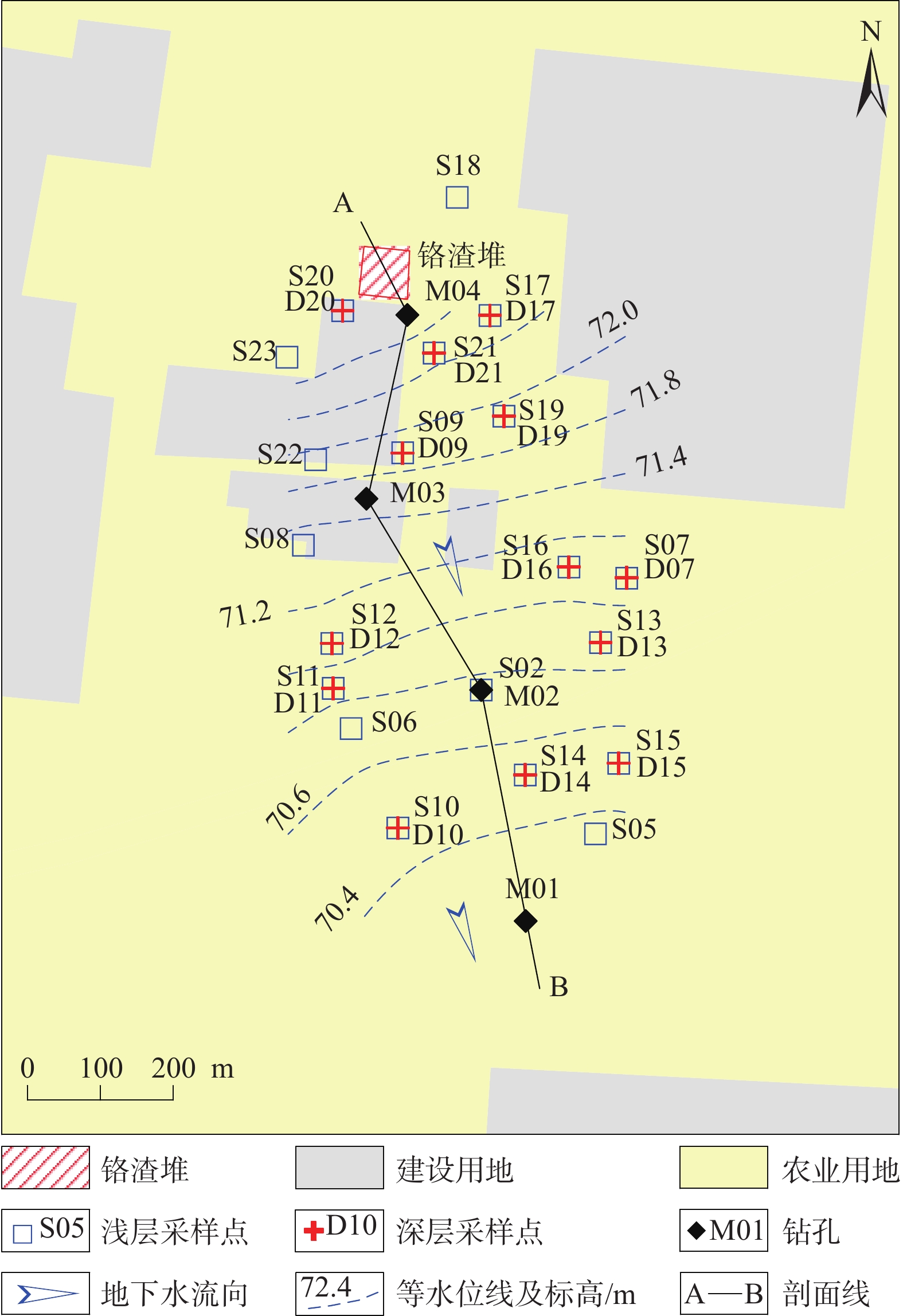
表 1 研究区地下水化学性质与特征污染物质量浓度统计表
Table 1. Statistical table of groundwater components and characteristic pollutants mass concentration
层
位统计值 pH ORP TDS TOC 组分质量浓度 K+ Na+ Ca2+ Mg2+ ${\mathrm{SO}}_4^{2-} $ Cl− ${\mathrm{HCO}}_3^- $ Fe Mn Cr Cr6+ 第
Ⅰ
含
水
层最小值 6.89 47.1 708 1.0 0.22 64.5 90.6 65.0 90.9 56.1 418 ND ND ND ND 最大值 7.73 165.5 1620 9.9 3.68 288.0 222.0 138.0 440.0 242.0 817 18.1 2.89 82.1 82.1 平均值 7.27 97.6 1191 2.2 0.89 135.2 140.7 99.2 210.8 156.5 564 1.7 0.44 4.0 4.0 变异系数 0.03 0.32 0.22 0.89 0.80 0.36 0.25 0.22 0.44 0.34 0.23 2.28 1.78 5.75 5.75 第
Ⅱ
含
水
层最小值 7.24 37.2 607 0.6 0.22 41.5 37.7 23.9 60.6 37.8 393 ND ND ND ND 最大值 8.08 158.7 1260 2.4 1.51 189.0 154.0 103.0 198.0 211.0 656 2.8 0.10 21.7 21.5 平均值 7.64 90.3 854 1.3 0.66 118.0 89.5 63.2 115.4 93.1 513 0.6 0.02 1.9 1.9 变异系数 0.04 0.41 0.26 0.46 0.57 0.39 0.39 0.34 0.41 0.59 0.16 1.25 1.82 3.09 3.11 注:表中变异系数和pH为无量纲;ORP单位为mV;其余指标单位为mg/L。 表 2 研究区旋转因子载荷矩阵
Table 2. Matrix of rotated factor loadings
组分 主因子 F1 F2 F3 F4 F5 pH 0.159 −0.108 0.694 −0.462 0.036 ORP 0.430 0.314 −0.003 0.693 −0.097 K+ 0.104 0.324 0.020 −0.121 0.189 Na+ 0.590 0.292 0.544 −0.065 0.344 Ca2+ 0.201 0.881 0.049 0.248 −0.080 Mg2+ 0.181 0.925 −0.058 0.182 −0.082 ${\mathrm{SO}}_4^{2-} $ 0.700 0.597 0.019 0.052 0.189 Cl− −0.071 0.915 0.168 −0.084 −0.146 ${\mathrm{HCO}}_3^- $ 0.020 0.166 0.446 0.519 0.568 TDS 0.415 0.846 0.177 −0.024 0.143 Fe −0.037 −0.026 0.013 0.981 0.023 Mn −0.129 0.347 0.166 0.703 0.294 TOC 0.102 0.193 0.204 0.925 0.093 总Cr 0.980 0.128 0.030 −0.075 −0.023 Cr(Ⅵ) 0.981 0.128 0.030 −0.074 −0.023 特征值 5.98 5.50 3.39 2.17 1.43 贡献率% 27.18 24.99 15.42 9.91 6.51 累计贡献率% 27.18 52.17 67.59 77.50 84.01 -
[1] WEI Yaqiang,XU Xiaoyun,ZHAO Ling,et al. Migration and transformation of chromium in unsaturated soil during groundwater table fluctuations induced by rainfall[J]. Journal of Hazardous Materials,2021,416:126229. doi: 10.1016/j.jhazmat.2021.126229
[2] 孟凡生. 中国铬渣污染场地土壤污染特征[J]. 环境污染与防治,2016,38(6):50 − 53. [MENG Fansheng. Pollution charateristics of soils polluted by chromium slag in China[J]. Environmental Pollution & Control,2016,38(6):50 − 53. (in Chinese with English abstract)]
MENG Fansheng. Pollution charateristics of soils polluted by chromium slag in China[J]. Environmental Pollution & Control, 2016, 38(6): 50 − 53. (in Chinese with English abstract)
[3] 王兴润,李磊,颜湘华,等. 铬污染场地修复技术进展[J]. 环境工程,2020,38(6):1 − 8. [WANG Xingrun,LI Lei,YAN Xianghua,et al. Progress in remediation of chromium-contaminated sites[J]. Environmental Engineering,2020,38(6):1 − 8. (in Chinese with English abstract)]
WANG Xingrun, LI Lei, YAN Xianghua, et al. Progress in remediation of chromium-contaminated sites[J]. Environmental Engineering, 2020, 38(6): 1 − 8. (in Chinese with English abstract)
[4] 刘玉强,李丽,王琪,等. 典型铬渣污染场地的污染状况与综合整治对策[J]. 环境科学研究,2009,22(2):248 − 253. [LIU Yuqiang,LI Li,WANG Qi,et al. Study on pollution situation at typical chrome residue contaminated sites and corresponding integrated remediation plan[J]. Research of Environmental Sciences,2009,22(2):248 − 253. (in Chinese with English abstract)]
LIU Yuqiang, LI Li, WANG Qi, et al. Study on pollution situation at typical chrome residue contaminated sites and corresponding integrated remediation plan[J]. Research of Environmental Sciences, 2009, 22(2): 248 − 253. (in Chinese with English abstract)
[5] 张树龙,张焕祯,王智丽,等. 铬盐清洁生产工艺研究进展[J]. 无机盐工业,2014,46(2):6 − 9. [ZHANG Shulong,ZHANG Huanzhen,WANG Zhili,et al. Research progress of cleaner production technology of chromate[J]. Inorganic Chemicals Industry,2014,46(2):6 − 9. (in Chinese with English abstract)] doi: 10.3969/j.issn.1006-4990.2014.02.002
ZHANG Shulong, ZHANG Huanzhen, WANG Zhili, et al. Research progress of cleaner production technology of chromate[J]. Inorganic Chemicals Industry, 2014, 46(2): 6 − 9. (in Chinese with English abstract) doi: 10.3969/j.issn.1006-4990.2014.02.002
[6] SHAHID M,SHAMSHAD S,RAFIQ M,et al. Chromium speciation,bioavailability,uptake,toxicity and detoxification in soil-plant system:A review[J]. Chemosphere,2017,178:513 − 533. doi: 10.1016/j.chemosphere.2017.03.074
[7] PRASAD S,YADAV K K,KUMAR S,et al. Chromium contamination and effect on environmental health and its remediation:A sustainable approaches[J]. Journal of Environmental Management,2021,285:112174. doi: 10.1016/j.jenvman.2021.112174
[8] WANG Xingrun,LI Lei,YAN Xianghua,et al. Processes of chromium (VI) migration and transformation in chromate production site:A case study from the middle of China[J]. Chemosphere,2020,257:127282. doi: 10.1016/j.chemosphere.2020.127282
[9] FU Yu,WANG Lingli,PENG Wenya,et al. Enabling simultaneous redox transformation of toxic chromium(VI) and arsenic(III) in aqueous media-a review[J]. Journal of Hazardous Materials,2021,417:126041. doi: 10.1016/j.jhazmat.2021.126041
[10] XU Teng,NAN Feng,JIANG Xiaofeng,et al. Effect of soil pH on the transport,fractionation,and oxidation of chromium(III)[J]. Ecotoxicology and Environmental Safety,2020,195:110459. doi: 10.1016/j.ecoenv.2020.110459
[11] 刘云惠,魏显有,王秀敏,等. 土壤中铬的吸附与形态提取研究[J]. 河北农业大学学报,2000,23(1):16 − 20. [LIU Yunhui,WEI Xianyou,WANG Xiumin,et al. A study on the adsorption of chromium in soil and the form extraction[J]. Journal of Agricultural University of Hebei,2000,23(1):16 − 20. (in Chinese with English abstract)] doi: 10.3969/j.issn.1000-1573.2000.01.005
LIU Yunhui, WEI Xianyou, WANG Xiumin, et al. A study on the adsorption of chromium in soil and the form extraction[J]. Journal of Agricultural University of Hebei, 2000, 23(1): 16 − 20. (in Chinese with English abstract) doi: 10.3969/j.issn.1000-1573.2000.01.005
[12] ZHANG Wenjie,LIN Mingfeng. Influence of redox potential on leaching behavior of a solidified chromium contaminated soil[J]. The Science of the Total Environment,2020,733:139410. doi: 10.1016/j.scitotenv.2020.139410
[13] 王成文,许模,张俊杰,等. 土壤pH和Eh对重金属铬(Ⅵ)纵向迁移及转化的影响[J]. 环境工程学报,2016,10(10):6035 − 6041. [WANG Chengwen,XU Mo,ZHANG Junjie,et al. Influence of soils pH and Eh on vertical migration and transformation of Cr(Ⅵ)[J]. Chinese Journal of Environmental Engineering,2016,10(10):6035 − 6041. (in Chinese with English abstract)] doi: 10.12030/j.cjee.201505010
WANG Chengwen, XU Mo, ZHANG Junjie, et al. Influence of soils pH and Eh on vertical migration and transformation of Cr(Ⅵ)[J]. Chinese Journal of Environmental Engineering, 2016, 10(10): 6035 − 6041. (in Chinese with English abstract) doi: 10.12030/j.cjee.201505010
[14] 孙亚乔,校康,段磊,等. 粘土矿物作用下铬的迁移转化机理研究进展[J]. 生态环境学报,2019,28(7):1484 − 1491. [SUN Yaqiao,XIAO Kang,DUAN Lei,et al. Chromium migration and transformation mechanism in the presence of clay minerals:A review[J]. Ecology and Environmental Sciences,2019,28(7):1484 − 1491. (in Chinese with English abstract)]
SUN Yaqiao, XIAO Kang, DUAN Lei, et al. Chromium migration and transformation mechanism in the presence of clay minerals: A review[J]. Ecology and Environmental Sciences, 2019, 28(7): 1484 − 1491. (in Chinese with English abstract)
[15] KWAK S,YOO J C,MOON D H,et al. Role of clay minerals on reduction of Cr(VI)[J]. Geoderma,2018,312:1 − 5. doi: 10.1016/j.geoderma.2017.10.001
[16] JAYACHANDRAN S,CHAKRABORTY P,SARKAR A,et al. Post depositional changes of sedimentary organic matter influence chromium speciation in continental slope sediments :A case study[J]. The Science of the Total Environment,2021,777:145783. doi: 10.1016/j.scitotenv.2021.145783
[17] SHI Zhenqing,PENG Shimeng,LIN Xiaofeng,et al. Predicting Cr(VI) adsorption on soils:The role of the competition of soil organic matter[J]. Environmental Science:Processes & Impacts,2020,22(1):95 − 104.
[18] 邹韵. 铬盐生产污染场地含水层水—岩界面铬迁移转化研究[D]. 北京:中国地质大学(北京),2015. [ZOU Yun. Study on chromium migration and transformation ingroundwater-rock interface in the chromium salt production contaminated sites[D]. Beijing:China University of Geosciences,2015. (in Chinese with English abstract)]
ZOU Yun. Study on chromium migration and transformation ingroundwater-rock interface in the chromium salt production contaminated sites[D]. Beijing: China University of Geosciences, 2015. (in Chinese with English abstract)
[19] ZHAO Xingmin,SOBECKY P A,ZHAO Lanpo,et al. Chromium(VI) transport and fate in unsaturated zone and aquifer:3D sandbox results[J]. Journal of Hazardous Materials,2016,306:203 − 209. doi: 10.1016/j.jhazmat.2015.12.004
[20] ZHANG Xiaowei,TONG Juxiu,HU B X,et al. Adsorption and desorption for dynamics transport of hexavalent chromium (Cr(VI)) in soil column[J]. Environmental Science and Pollution Research International,2018,25(1):459 − 468. doi: 10.1007/s11356-017-0263-0
[21] 贺勇,胡广,张召,等. 污染场地六价铬迁移转化机制与数值模拟研究[J]. 岩土力学,2022,43(2):528 − 538. [HE Yong,HU Guang,ZHANG Zhao,et al. Numerical simulation on the migration and transformation mechanism of hexavalent chromium in contaminated site[J]. Rock and Soil Mechanics,2022,43(2):528 − 538. (in Chinese with English abstract)]
HE Yong, HU Guang, ZHANG Zhao, et al. Numerical simulation on the migration and transformation mechanism of hexavalent chromium in contaminated site[J]. Rock and Soil Mechanics, 2022, 43(2): 528 − 538. (in Chinese with English abstract)
[22] 刘玲,肖利萍,李喜林. 铬渣渗滤液中六价铬在地下水中迁移模拟[J]. 系统仿真学报,2018,30(2):560 − 568. [LIU Ling,XIAO Liping,LI Xilin. Simulation of migration of hexavalent chromium in groundwater[J]. Journal of System Simulation,2018,30(2):560 − 568. (in Chinese with English abstract)]
LIU Ling, XIAO Liping, LI Xilin. Simulation of migration of hexavalent chromium in groundwater[J]. Journal of System Simulation, 2018, 30(2): 560 − 568. (in Chinese with English abstract)
[23] 吕永高,蔡五田,杨骊,等. 中试尺度下可渗透反应墙位置优化模拟——以铬污染地下水场地为例[J]. 水文地质工程地质,2020,47(5):189 − 195. [LYU Yonggao,CAI Wutian,YANG Li,et al. A numerical simulation study of the position optimization of a pilot-scale permeable reactive barrier:A case study of the hexavalent chromium contaminated site[J]. Hydrogeology & Engineering Geology,2020,47(5):189 − 195. (in Chinese with English abstract)]
LYU Yonggao, CAI Wutian, YANG Li, et al. A numerical simulation study of the position optimization of a pilot-scale permeable reactive barrier: A case study of the hexavalent chromium contaminated site[J]. Hydrogeology & Engineering Geology, 2020, 47(5): 189 − 195. (in Chinese with English abstract)
[24] 边超,蔡五田,吕永高,等. Cr(Ⅵ)踪迹指标现场测定用于铬污染地下水源解析[J]. 环境科学与技术,2020,43(2):150 − 155. [BIAN Chao,CAI Wutian,LYU Yonggao,et al. Field determination of trace ion in Cr(Ⅵ)-polluted groundwater and its application in source apportionment[J]. Environmental Science & Technology,2020,43(2):150 − 155. (in Chinese with English abstract)]
BIAN Chao, CAI Wutian, LYU Yonggao, et al. Field determination of trace ion in Cr(Ⅵ)-polluted groundwater and its application in source apportionment[J]. Environmental Science & Technology, 2020, 43(2): 150 − 155. (in Chinese with English abstract)
[25] 边超,蔡五田,刘金巍,等. FPXRF用于污染场地铬分布特征及迁移规律研究[J]. 环境科学与技术,2017,40(12):126 − 132. [BIAN Chao,CAI Wutian,LIU Jinwei,et al. Study on distribution and migration of chromium in contaminated site with FPXRF[J]. Environmental Science & Technology,2017,40(12):126 − 132. (in Chinese with English abstract)]
BIAN Chao, CAI Wutian, LIU Jinwei, et al. Study on distribution and migration of chromium in contaminated site with FPXRF[J]. Environmental Science & Technology, 2017, 40(12): 126 − 132. (in Chinese with English abstract)
[26] 边超,蔡五田,刘金巍,等. 土壤中Cr(Ⅵ)现场检测技术在污染调查中的应用[J]. 环境科学与技术,2016,39(5):53 − 55. [BIAN Chao,CAI Wutian,LIU Jinwei,et al. Application of in situ detecting technique for Cr(Ⅵ) in polluted soil investigation[J]. Environmental Science & Technology,2016,39(5):53 − 55. (in Chinese with English abstract)]
BIAN Chao, CAI Wutian, LIU Jinwei, et al. Application of in situ detecting technique for Cr(Ⅵ) in polluted soil investigation[J]. Environmental Science & Technology, 2016, 39(5): 53 − 55. (in Chinese with English abstract)
[27] CHEN Wenfang,ZHANG Yaobin,SHI Weiwei,et al. Analysis of hydrogeochemical characteristics and origins of chromium contamination in groundwater at a site in Xinxiang City,Henan Province[J]. Applied Sciences,2021,11(24):11683.
[28] ZHANG Yaobin,ZHANG Qiulan,CHEN Wenfang,et al. Hydrogeochemical analysis and groundwater pollution source identification based on self-organizing map at a contaminated site[J]. Journal of Hydrology,2023,616:128839.
[29] 国家质量监督检验检疫总局,中国国家标准化管理委员会. 地下水质量标准:GB/T 14848—2017[S]. 北京:中国标准出版社,2017. [General Administration of Quality Supervision,Inspection and Quarantine of the People’s Republic of China,Standardization Administration of the People’s Republic of China. Standard for groundwater quality:GB/T 14848—2017[S]. Beijing:Standards Press of China,2017. (in Chinese)]
General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Standard for groundwater quality: GB/T 14848—2017[S]. Beijing: Standards Press of China, 2017. (in Chinese)
[30] 吕航,刘明遥,苏小四,等. 主因子分析方法在确定地下水石油烃生物降解过程中的应用[J]. 中南大学学报(自然科学版),2013,44(8):3552 − 3560. [LYU Hang,LIU Mingyao,SU Xiaosi,et al. Determination of biogeochemical processes in oil-contaminated aquifer using principal component analysis[J]. Journal of Central South University (Science and Technology),2013,44(8):3552 − 3560. (in Chinese with English abstract)]
LYU Hang, LIU Mingyao, SU Xiaosi, et al. Determination of biogeochemical processes in oil-contaminated aquifer using principal component analysis[J]. Journal of Central South University (Science and Technology), 2013, 44(8): 3552 − 3560. (in Chinese with English abstract)
[31] 钱康,张继,陈鹏,等. 云南乌蒙山盘河地区地下水水化学及同位素特征[J]. 地质通报,2022,41(7):1291 − 1299. [QIAN Kang,ZHANG Ji,CHEN Peng,et al. Hydrochemical and isotopic characteristics of groundwater in Panhe Area of Wumeng Mountain,Yunnan[J]. Geological Bulletin of China,2022,41(7):1291 − 1299. (in Chinese with English abstract)] doi: 10.12097/j.issn.1671-2552.2022.07.015
QIAN Kang, ZHANG Ji, CHEN Peng, et al. Hydrochemical and isotopic characteristics of groundwater in Panhe Area of Wumeng Mountain, Yunnan[J]. Geological Bulletin of China, 2022, 41(7): 1291 − 1299. (in Chinese with English abstract) doi: 10.12097/j.issn.1671-2552.2022.07.015
[32] BETHKE C M. Geochemical and Biogeochemical Reaction Modeling[M]. Cambridge,UK:Cambridge University Press,2007.
[33] 丁翼. 中国铬盐生产状况与展望[J]. 化工进展,2004,23(4):345 − 348. [DING Yi. Present status and prospect of chromate production in China[J]. Chemical Industry and Engineering Progress,2004,23(4):345 − 348. (in Chinese with English abstract)] doi: 10.3321/j.issn:1000-6613.2004.04.002
DING Yi. Present status and prospect of chromate production in China[J]. Chemical Industry and Engineering Progress, 2004, 23(4): 345 − 348. (in Chinese with English abstract) doi: 10.3321/j.issn:1000-6613.2004.04.002
[34] 崔佳琪,李仙岳,史海滨,等. 河套灌区地下水化学演变特征及形成机制[J]. 环境科学,2020,41(9):4011 − 4020. [CUI Jiaqi,LI Xianyue,SHI Haibin,et al. Chemical evolution and formation mechanism of groundwater in Hetao irrigation area[J]. Environmental Science,2020,41(9):4011 − 4020. (in Chinese with English abstract)]
CUI Jiaqi, LI Xianyue, SHI Haibin, et al. Chemical evolution and formation mechanism of groundwater in Hetao irrigation area[J]. Environmental Science, 2020, 41(9): 4011 − 4020. (in Chinese with English abstract)
[35] 李状,苏晶文,董长春,等. 安徽马鞍山市当涂地区地下水水化学特征及演化机制[J]. 中国地质,2022,49(5):1509 − 1526. [LI Zhuang,SU Jingwen,DONG Changchun,et al. Hydrochemistry characteristics and evolution mechanisms of the groundwater in Dangtu Area,Ma’anshan City,Anhui Province[J]. Geology in China,2022,49(5):1509 − 1526. ( in Chinese with English abstract] doi: 10.12029/gc20220510
LI Zhuang, SU Jingwen, DONG Changchun, et al. Hydrochemistry characteristics and evolution mechanisms of the groundwater in Dangtu Area, Ma’anshan City, Anhui Province[J]. Geology in China, 2022, 49(5): 1509 − 1526. ( in Chinese with English abstract doi: 10.12029/gc20220510
[36] 李志红,王广才,康飞,等. 基于水化学和同位素特征的新乡某地下水污染场地水文地质概念模型细化[J]. 水文地质工程地质,2017,44(2):57 − 62. [LI Zhihong,WANG Guangcai,KANG Fei,et al. Boundary refine of hydrogeological conceptional model of a groundwater contaminated site in Xinxiang city based on the hydrochemistry and isotope evidence[J]. Hydrogeology & Engineering Geology,2017,44(2):57 − 62. (in Chinese with English abstract)]
LI Zhihong, WANG Guangcai, KANG Fei, et al. Boundary refine of hydrogeological conceptional model of a groundwater contaminated site in Xinxiang city based on the hydrochemistry and isotope evidence[J]. Hydrogeology & Engineering Geology, 2017, 44(2): 57 − 62. (in Chinese with English abstract)
[37] 冯国平. 豫北山前新乡市北部冲洪积扇地下水水化学特征及演化机理研究[D]. 青岛:山东科技大学,2020. [FENG Guoping. Research on groundwater hydrochemical characteristics and evolution mechanism of alluvial fans in the north of Xinxiang City,piedmont of northern Henan[D]. Qingdao:Shandong University of Science and Technology,2020. (in Chinese with English abstract)]
FENG Guoping. Research on groundwater hydrochemical characteristics and evolution mechanism of alluvial fans in the north of Xinxiang City, piedmont of northern Henan[D]. Qingdao: Shandong University of Science and Technology, 2020. (in Chinese with English abstract)
[38] FAN Xianfang,DING Shiming,CHEN Musong,et al. Mobility of chromium in sediments dominated by macrophytes and cyanobacteria in different zones of Lake Taihu[J]. The Science of the Total Environment,2019,666:994-1002. [PubMed]
[39] 白利平,王业耀. 铬在土壤及地下水中迁移转化研究综述[J]. 地质与资源,2009,18(2):144 − 148. [BAI Liping,WANG Yeyao. Research progress of chromium disposition and distribution in soil and groundwater[J]. Geology and Resources,2009,18(2):144 − 148. (in Chinese with English abstract)] doi: 10.3969/j.issn.1671-1947.2009.02.014
BAI Liping, WANG Yeyao. Research progress of chromium disposition and distribution in soil and groundwater[J]. Geology and Resources, 2009, 18(2): 144 − 148. (in Chinese with English abstract) doi: 10.3969/j.issn.1671-1947.2009.02.014
[40] JOBBY R,JHA P,YADAV A K,et al. Biosorption and biotransformation of hexavalent chromium[Cr(VI)]:A comprehensive review[J]. Chemosphere,2018,207:255 − 266. doi: 10.1016/j.chemosphere.2018.05.050
[41] 陈英旭,何增耀,吴建平. 土壤中铬的形态及其转化[J]. 环境科学,1994,15(3):53 − 56. [CHEN Yingxu,HE Zengyao,WU Jianping. Forms and transformation of chromium species in soils[J]. Environmental Science,1994,15(3):53 − 56. (in Chinese)] doi: 10.3321/j.issn:0250-3301.1994.03.022
CHEN Yingxu, HE Zengyao, WU Jianping. Forms and transformation of chromium species in soils[J]. Environmental Science, 1994, 15(3): 53 − 56. (in Chinese) doi: 10.3321/j.issn:0250-3301.1994.03.022
[42] 桂新安,杨海真,王少平,等. 铬在土壤中的吸附解吸研究进展[J]. 土壤通报,2007,38(5):1007 − 1012. [GUI Xin’an,YANG Haizhen,WANG Shaoping,et al. Advance in studies of chromium sorption and desorption in soils[J]. Chinese Journal of Soil Science,2007,38(5):1007 − 1012. (in Chinese with English abstract)]
GUI Xin’an, YANG Haizhen, WANG Shaoping, et al. Advance in studies of chromium sorption and desorption in soils[J]. Chinese Journal of Soil Science, 2007, 38(5): 1007 − 1012. (in Chinese with English abstract)
[43] BAE S,YOON S,KAPLAN U,et al. Effect of groundwater ions (Ca2+,Na+,and ${\mathrm{HCO}}_3^- $) on removal of hexavalent chromium by Fe(II)-phosphate mineral[J]. Journal of Hazardous Materials,2020,398:122948. doi: 10.1016/j.jhazmat.2020.122948
-



 下载:
下载: