Geochemical records of biological carriers on deepsea hydrothermal vent and methane seep fields
-
摘要:
海底冷泉-热液极端环境是岩石圈与外部圈层进行物质交换和能量流动的主要窗口,其独特的地质条件和营养模式孕育了繁茂的生物群落和生态系统。由于多种因素的叠加控制,海底极端环境的理化性质通常变化频繁且剧烈。大量研究表明,这种变化或波动又能不同程度地被环境中生存的生物记录下来,因此,生物所保留下来的某些地球化学信息具有恢复重建其生存环境变化的潜在能力,这对人类目前尚难自由出入的深海极端环境的探索尤为重要。本文从海底极端环境生物种类和空间分布、生物壳体的地球化学信息、生物元素和同位素地球化学指标以及生物有机生标等几方面出发,探讨当前科学家关注的典型地球化学指标对沉积环境的记录应用,并展望了未来需要进一步加强研究的几个方面,希望借此能引起广大研究者对该领域的兴趣和重视,以加深加快对海底极端环境内运行规律和影响的认识。
Abstract:The environments of cold seep and hydrothermal vent are the main windows for material exchange and energy flow between lithosphere and outer spheres, and their unique geological conditions and nutrient patterns give birth to the lush biological communities and ecosystems. The change of physical and chemical properties of extreme environment will affect the geochemical information carried by the organisms living in extreme environment, so the extreme environment organisms could be used as a effective proxy for environmental recovery. In this paper, attempt is made to address the concerned geochemical information with emphases on biological species, spatial distribution pattern, mineral geochemistry of biocarrier rocks, geochemical indicators of biological elements and isotopes, and lipid biomarker in paleoenvironmental reconstruction. The application of the information to the restoration of sedimentary environments is discussed, in addition to future research directions. We hope that the introduction may raise interests and attentions from researchers.
-
Key words:
- hydrothermal vent /
- cold seeps /
- biological carriers /
- geochemical records /
- isotopic records /
- organic biomarkers
-

-
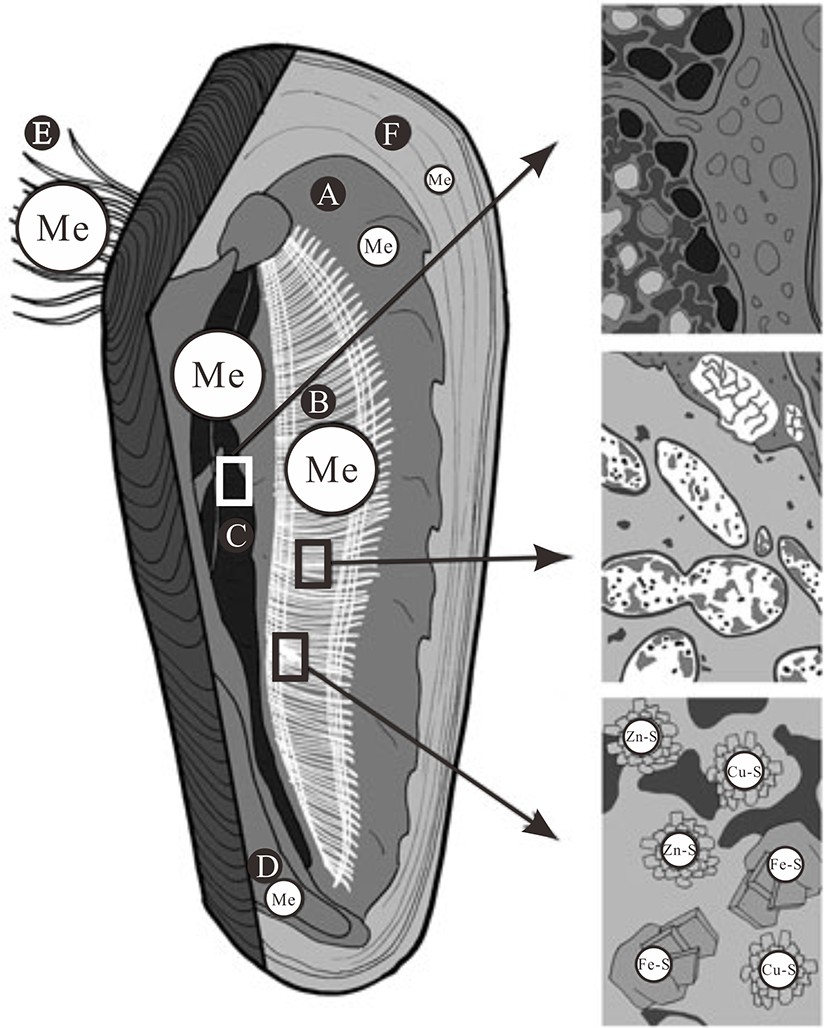
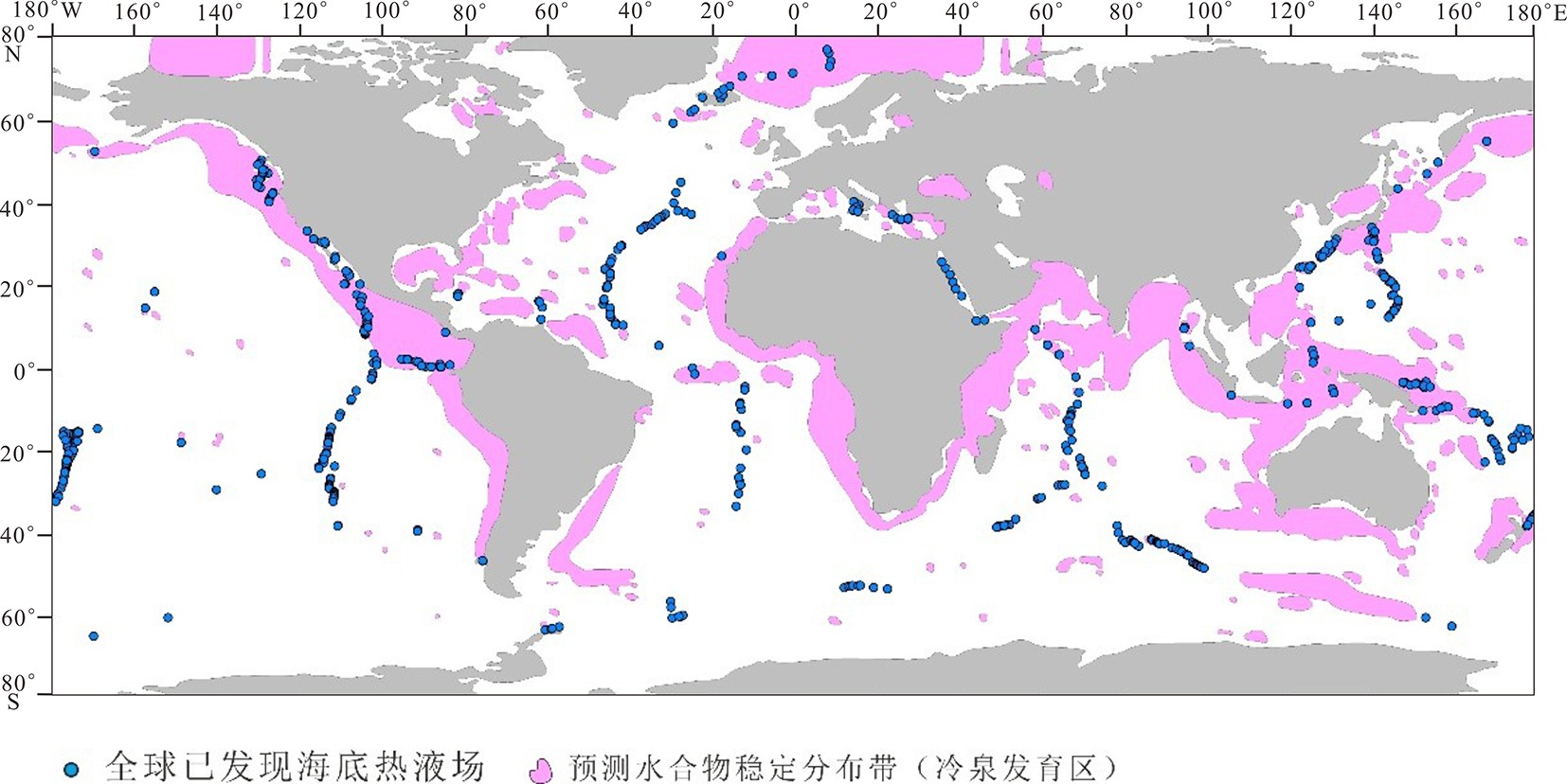
图 1 世界海洋中已发现热液喷口和可能冷泉发育区叠置图 [3]
Figure 1.
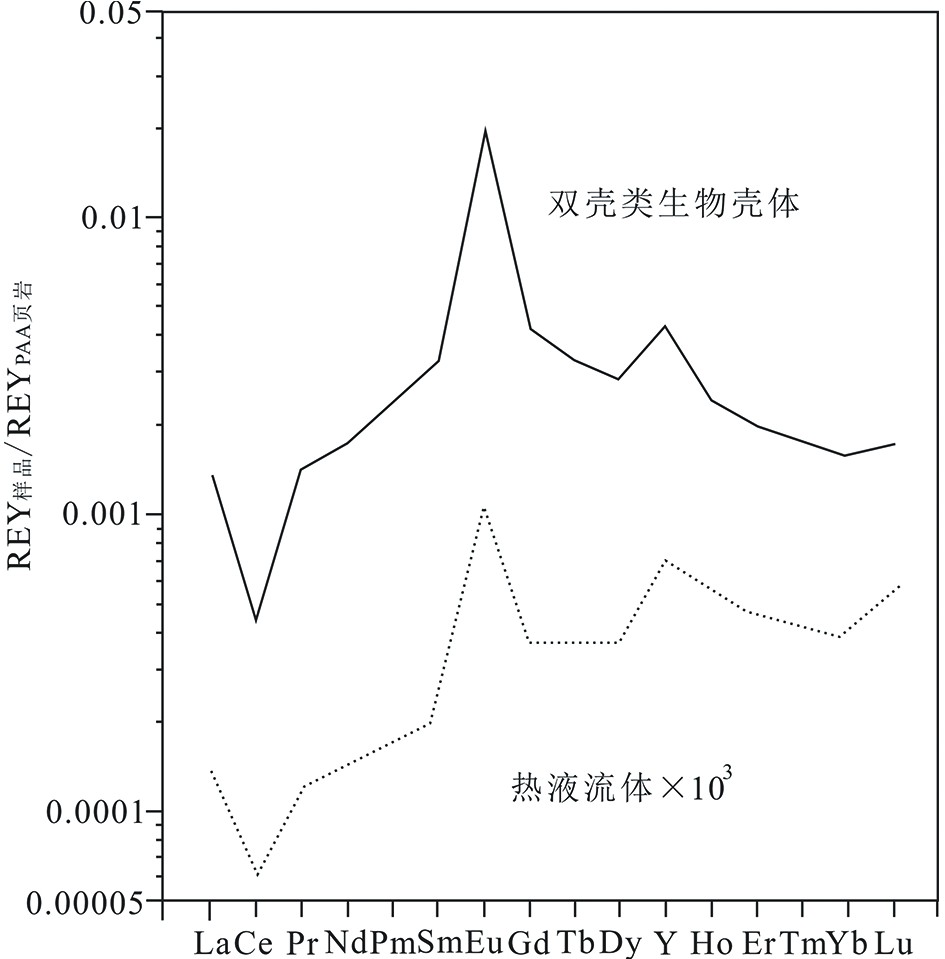
图 3 热液区贻贝壳体和热液稀土模式分布图[47]
Figure 3.
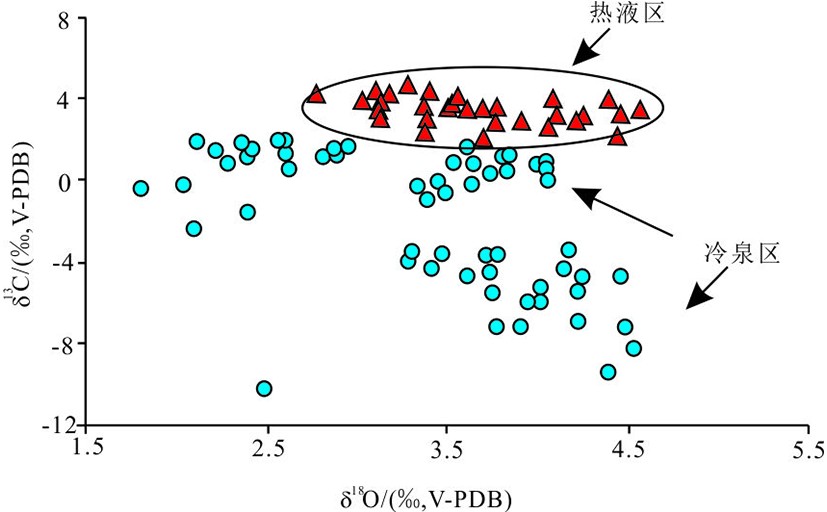
图 4 冷泉区和热液区生物壳体碳-氧同位素分布图[68]
Figure 4.
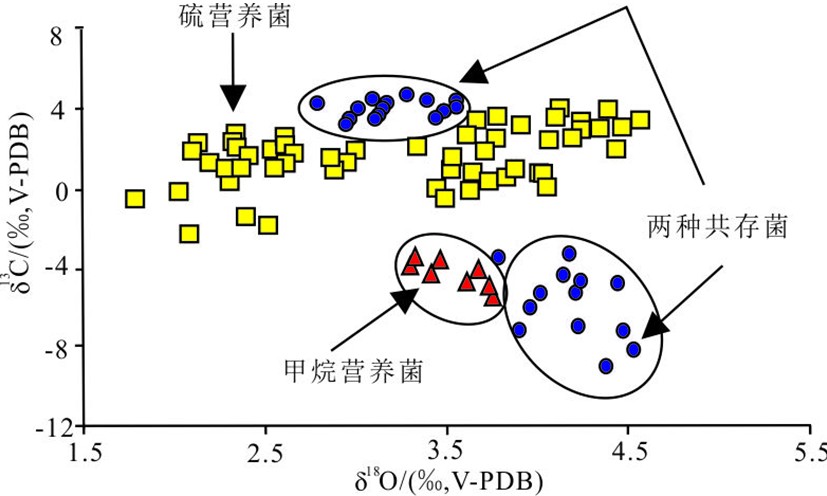
图 5 不同共生菌的生物壳体碳氧同位素分布图[68]
Figure 5.
表 1 海底冷泉和热液系统宏体生物的地球化学记录特征对比
Table 1. Comparison of geochemical record characteristics of macrobiota of seafloor cold seeps and hydrothermal systems
相似点流体浓度决定生物种类和数量 记录栖息地地球化学特征 生物体不同组织的元素种类和含量不同 微生物过程和周围环境影响生物同位素信息
不同点热液区生物种类和数量受温度影响较大 热液区生物的金属元素含量更高 稀土元素的富集模式不同 生物标志物种类不同 -
[1] 魏合龙, 孙治雷, 王利波, 等. 天然气水合物系统的环境效应[J]. 海洋地质与第四纪地质, 2016, 36(1):1-13
WEI Helong, SUN Zhilei, WANG Libo, et al. Perspective of the environmental effect of natural gas hydrate system [J]. Marine Geology & Quaternary Geology, 2016, 36(1): 1-13.
[2] 席世川, 张鑫, 王冰, 等. 海底冷泉标志与主要冷泉区的分布和比较[J]. 海洋地质前沿, 2017, 33(2):7-18
XI Shichuan, ZHANG Xin, WANG Bing, et al. The indicators of seabed cold seep and comparison among main distribution areas [J]. Marine Geology Frontiers, 2017, 33(2): 7-18.
[3] Sun Z L, Wu N Y, Cao H, et al. Hydrothermal metal supplies enhance the benthic methane filter in oceans: an example from the Okinawa Trough [J]. Chemical Geology, 2019, 525: 190-209. doi: 10.1016/j.chemgeo.2019.07.025
[4] Nakajima Y, Shinzato C, Khalturina M, et al. Isolation and characterization of novel polymorphic microsatellite loci for the deep-sea hydrothermal vent limpet, Lepetodrilus nux, and the vent-associated squat lobster, Shinkaia crosnieri [J]. Marine Biodiversity, 2018, 48(1): 677-684. doi: 10.1007/s12526-017-0704-5
[5] Parson L M, Walker C L, Dixon D R. Hydrothermal vents and processes [J]. Geological Society, London, Special Publications, 1995, 87: 1-2. doi: 10.1144/GSL.SP.1995.087.01.01
[6] German C R, Seyfried W E Jr. Hydrothermal processes [J]. Treatise on Geochemistry, 2014, 6: 191-233.
[7] Cardigos F, Colaço A, Dando P R, et al. Shallow water hydrothermal vent field fluids and communities of the D. João de Castro Seamount (Azores) [J]. Chemical Geology, 2005, 224(1-3): 153-168. doi: 10.1016/j.chemgeo.2005.07.019
[8] Melwani A R, Kim S L. Benthic infaunal distributions in shallow hydrothermal vent sediments [J]. Acta Oecologica, 2008, 33(2): 162-175. doi: 10.1016/j.actao.2007.10.008
[9] Dando P R. Biological communities at marine shallow-water vent and seep sites[M]//Kiel S. The Vent and Seep Biota. Dordrecht: Springer, 2010: 333-378.
[10] Markulin K, Peharda M, Mertz-Kraus R, et al. Trace and minor element records in aragonitic bivalve shells as environmental proxies [J]. Chemical Geology, 2019, 507: 120-133. doi: 10.1016/j.chemgeo.2019.01.008
[11] Galkin S V. Structure of hydrothermal vent communities[M]//Demina L L, Galkin S V. Trace Metal Biogeochemistry and Ecology of Deep-Sea Hydrothermal Vent Systems. Cham: Springer, 2016: 77-95.
[12] Schreier J E, Lutz R A. Hydrothermal vent biota[M]//Steele J H. Encyclopedia of Ocean Sciences. Elsevier: Academic Press, 2019: 308-319.
[13] Dattagupta S, Arthur M A, Fisher C R. Modification of sediment geochemistry by the hydrocarbon seep tubeworm Lamellibrachia luymesi: a combined empirical and modeling approach [J]. Geochimica et Cosmochimica Acta, 2008, 72(9): 2298-2315. doi: 10.1016/j.gca.2008.02.016
[14] Taviani M, Angeletti L, Ceregato A, et al. The Gela Basin pockmark field in the strait of Sicily (Mediterranean Sea): chemosymbiotic faunal and carbonate signatures of postglacial to modern cold seepage [J]. Biogeosciences, 2013, 10(7): 4653-4671. doi: 10.5194/bg-10-4653-2013
[15] Lenihan H S, Mills S W, Mullineaux L S, et al. Biotic interactions at hydrothermal vents: recruitment inhibition by the mussel Bathymodiolus thermophilus [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2008, 55(12): 1707-1717. doi: 10.1016/j.dsr.2008.07.007
[16] Karpen V, Thomsen L, Suess E. Groundwater discharges in the Baltic Sea: survey and quantification using a schlieren technique application [J]. Geofluids, 2010, 6(3): 241-250.
[17] Jensen P, Aagaard I, Burke R A Jr, et al. ‘Bubbling reefs’ in the Kattegat: submarine landscapes of carbonate-cemented rocks support a diverse ecosystem at methane seeps [J]. Marine Ecology Progress Series, 1992, 83: 103-112. doi: 10.3354/meps083103
[18] Snelgrove P V R. Hydrodynamic enhancement of invertebrate larval settlement in microdepositional environments: colonization tray experiments in a muddy habitat [J]. Journal of Experimental Marine Biology and Ecology, 1994, 176(2): 149-166. doi: 10.1016/0022-0981(94)90182-1
[19] Tarasov V G, Gebruk A V, Mironov A N, et al. Deep-sea and shallow-water hydrothermal vent communities: two different phenomena? [J]. Chemical Geology, 2005, 224(1-3): 5-39. doi: 10.1016/j.chemgeo.2005.07.021
[20] Mae A, Yamanaka T, Shimoyama S. Stable isotope evidence for identification of chemosynthesis-based fossil bivalves associated with cold-seepages [J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2007, 245(3-4): 411-420. doi: 10.1016/j.palaeo.2006.09.003
[21] Dando P R, Aliani S, Arab H, et al. Hydrothermal studies in the Aegean Sea [J]. Physics and Chemistry of the Earth, Part B:Hydrology, Oceans and Atmosphere, 2000, 25(1): 1-8. doi: 10.1016/S1464-1909(99)00112-4
[22] Beccari V, Basso D, Spezzaferri S, et al. Preliminary video-spatial analysis of cold seep bivalve beds at the base of the continental slope of Israel (Palmahim Disturbance) [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2020, 171: 104664. doi: 10.1016/j.dsr2.2019.104664
[23] Guillon E, Menot L, Decker C, et al. The vesicomyid bivalve habitat at cold seeps supports heterogeneous and dynamic macrofaunal assemblages [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2017, 120: 1-13. doi: 10.1016/j.dsr.2016.12.008
[24] Campbell K A, Bottjer D J. Brachiopods and chemosymbiotic bivalves in Phanerozoic hydrothermal vent and cold seep environments [J]. Geology, 1995, 23(4): 321-324. doi: 10.1130/0091-7613(1995)023<0321:BACBIP>2.3.CO;2
[25] Feng D, Roberts H H. Initial results of comparing cold-seep carbonates from mussel- and tubeworm-associated environments at Atwater Valley lease block 340, northern Gulf of Mexico [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2010, 57(21-23): 2030-2039. doi: 10.1016/j.dsr2.2010.05.004
[26] Pellerin A, Antler G, Røy H, et al. The sulfur cycle below the sulfate-methane transition of marine sediments [J]. Geochimica et Cosmochimica Acta, 2018, 239: 74-89. doi: 10.1016/j.gca.2018.07.027
[27] Duros P, Silva Jacinto R, Dennielou B, et al. Benthic foraminiferal response to sedimentary disturbance in the Capbreton canyon (Bay of Biscay, NE Atlantic) [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2017, 120: 61-75. doi: 10.1016/j.dsr.2016.11.012
[28] Khripounoff A, Caprais J C, Decker C, et al. Variability in gas and solute fluxes through deep-sea chemosynthetic ecosystems inhabited by vesicomyid bivalves in the Gulf of Guinea [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2015, 95: 122-130. doi: 10.1016/j.dsr.2014.10.013
[29] Marlow J J, Steele J A, Ziebis W, et al. Carbonate-hosted methanotrophy represents an unrecognized methane sink in the deep sea [J]. Nature Communications, 2014, 5(1): 5094. doi: 10.1038/ncomms6094
[30] Toyama K, Paytan A, Sawada K, et al. Sulfur isotope ratios in co-occurring barite and carbonate from Eocene sediments: a comparison study [J]. Chemical Geology, 2020, 535: 119454. doi: 10.1016/j.chemgeo.2019.119454
[31] Roberts H H, Feng D. Carbonate precipitation at gulf of mexico hydrocarbon seeps: an overview[M]//Aminzadeh F, Berge T B, Connolly D L. Hydrocarbon Seepage. American: Society of Exploration Geophysicists, 2013: 43-61.
[32] Scheller S, Yu H, Chadwick G L, et al. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction [J]. Science, 2016, 351(6274): 703-707. doi: 10.1126/science.aad7154
[33] Tong H P, Feng D, Cheng H, et al. Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology [J]. Marine and Petroleum Geology, 2013, 43: 260-271. doi: 10.1016/j.marpetgeo.2013.01.011
[34] Dattagupta S, Miles L L, Barnabei M S, et al. The hydrocarbon seep tubeworm Lamellibrachia luymesi primarily eliminates sulfate and hydrogen ions across its roots to conserve energy and ensure sulfide supply [J]. Journal of Experimental Biology, 2006, 209(19): 3795-3805. doi: 10.1242/jeb.02413
[35] Cordes E E, Becker E L, Hourdez S, et al. Influence of foundation species, depth, and location on diversity and community composition at Gulf of Mexico lower-slope cold seeps [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2010, 57(21-23): 1870-1881. doi: 10.1016/j.dsr2.2010.05.010
[36] Kádár E, Costa V. First report on the micro-essential metal concentrations in bivalve shells from deep-sea hydrothermal vents [J]. Journal of Sea Research, 2006, 56(1): 37-44. doi: 10.1016/j.seares.2006.01.001
[37] 冯东, 宫尚桂. 海底冷泉系统硫的生物地球化学过程及其沉积记录研究进展[J]. 矿物岩石地球化学通报, 2019, 38(6):1047-1056
FENG Dong, GONG Shanggui. Progress on the biogeochemical process of sulfur and its geological record at submarine cold seeps [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2019, 38(6): 1047-1056.
[38] Basso D, Beccari V, Almogi-Labin A, et al. Macro- and micro-fauna from cold seeps in the Palmahim Disturbance (Israeli off-shore), with description of Waisiuconcha corsellii n. sp. (Bivalvia, Vesicomyidae) [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2020, 171: 104723. doi: 10.1016/j.dsr2.2019.104723
[39] Zeng Z G, Chen S, Ma Y, et al. Chemical compositions of mussels and clams from the Tangyin and Yonaguni Knoll IV hydrothermal fields in the southwestern Okinawa Trough [J]. Ore Geology Reviews, 2017, 87: 172-191. doi: 10.1016/j.oregeorev.2016.09.015
[40] Amano K, Jenkins R G, Sako Y, et al. A Paleogene deep-sea methane-seep community from Honshu, Japan [J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2013, 387: 126-133. doi: 10.1016/j.palaeo.2013.07.015
[41] Pavlidou A, Velaoras D, Karageorgis A P, et al. Seasonal variations of biochemical and optical properties, physical dynamics and N stable isotopic composition in three northeastern Mediterranean basins (Aegean, Cretan and Ionian Seas) [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2020, 171: 104704. doi: 10.1016/j.dsr2.2019.104704
[42] Almeida M J, Machado J, Moura G, et al. Temporal and local variations in biochemical composition of Crassostrea gigas shells [J]. Journal of Sea Research, 1998, 40(3-4): 233-249. doi: 10.1016/S1385-1101(98)00033-1
[43] Wang X C, Li C L, Zhou L. Metal concentrations in the mussel Bathymodiolus platifrons from a cold seep in the South China Sea [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2017, 129: 80-88. doi: 10.1016/j.dsr.2017.10.004
[44] Ruelas-Inzunza J, Soto L A, Páez-Osuna F. Heavy-metal accumulation in the hydrothermal vent clam Vesicomya gigas from Guaymas basin, Gulf of California [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2003, 50(6): 757-761. doi: 10.1016/S0967-0637(03)00054-2
[45] Koschinsky A. Sources and forms of trace metals taken up by hydrothermal vent mussels, and possible adaption and mitigation strategies[M]//Demina L L, Galkin S V. Trace Metal Biogeochemistry and Ecology of Deep-Sea Hydrothermal Vent Systems. Cham: Springer, 2016: 97-122.
[46] Bau M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium [J]. Chemical Geology, 1991, 93(3-4): 219-230. doi: 10.1016/0009-2541(91)90115-8
[47] Bau M, Balan S, Schmidt K, et al. Rare earth elements in mussel shells of the Mytilidae family as tracers for hidden and fossil high-temperature hydrothermal systems [J]. Earth and Planetary Science Letters, 2010, 299(3-4): 310-316. doi: 10.1016/j.jpgl.2010.09.011
[48] 李景喜, 孙承君, 蒋凤华, 等. 印度洋热液区贻贝及栖息沉积物中金属元素的特征分析[J]. 分析化学, 2017, 45(9):1316-1322 doi: 10.11895/j.issn.0253-3820.170348
LI Jingxi, SUN Chengjun, JIANG Fenghua, et al. Characteristics analysis of metal elements in sediments and habitat mussels from india ocean hydrothermal area [J]. Chinese Journal of Analytical Chemistry, 2017, 45(9): 1316-1322. doi: 10.11895/j.issn.0253-3820.170348
[49] Vanreusel A, De Groote A, Gollner S, et al. Ecology and biogeography of free-living nematodes associated with chemosynthetic environments in the deep sea: a review [J]. PLoS One, 2010, 5(8): e12449. doi: 10.1371/journal.pone.0012449
[50] Shiller A M, Chan E W, Joung D J, et al. Light rare earth element depletion during Deepwater Horizon blowout methanotrophy [J]. Scientific Reports, 2017, 7(1): 10389. doi: 10.1038/s41598-017-11060-z
[51] Wang X, Barrat J A, Bayon G, et al. Lanthanum anomalies as fingerprints of methanotrophy [J]. Geochemical Perspectives Letters, 2020, 14: 26-30. doi: 10.7185/geochemlet.2019
[52] Akagi T, Edanami K. Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo Bay [J]. Marine Chemistry, 2017, 194: 55-62. doi: 10.1016/j.marchem.2017.02.009
[53] Yamanaka T, Mizota C, Fujiwara Y, et al. Sulphur-isotopic composition of the deep-sea mussel Bathymodiolus marisindicus from currently active hydrothermal vents in the Indian Ocean [J]. Journal of the Marine Biological Association of the United Kingdom, 2003, 83(4): 841-848. doi: 10.1017/S0025315403007872h
[54] Paull C K, Jull A J T, Toolin L J, et al. Stable isotope evidence for chemosynthesis in an abyssal seep community [J]. Nature, 1985, 317(6039): 709-711. doi: 10.1038/317709a0
[55] Suzuki Y, Sasaki T, Suzuki M, et al. Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean [J]. Applied and Environmental Microbiology, 2005, 71(9): 5440-5450. doi: 10.1128/AEM.71.9.5440-5450.2005
[56] Yamanaka T, Shimamura S, Nagashio H, et al. A compilation of the stable isotopic compositions of carbon, nitrogen, and sulfur in soft body parts of animals collected from deep-sea hydrothermal vent and methane seep fields: variations in energy source and importance of subsurface microbial processes in the sediment-hosted systems[M]//Ishibashi J I, Okino K, Sunamura M. Subseafloor Biosphere Linked to Hydrothermal Systems. Tokyo: Springer, 2015: 105-129.
[57] De Ronde C E J, Massoth G J, Butterfield D A, et al. Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand [J]. Mineralium Deposita, 2011, 46(5-6): 541-584. doi: 10.1007/s00126-011-0345-8
[58] Markert S, Arndt C, Felbeck H, et al. Physiological proteomics of the uncultured endosymbiont of Riftia pachyptila [J]. Science, 2007, 315(5809): 247-250. doi: 10.1126/science.1132913
[59] Bell J B, Reid W D K, Pearce D A, et al. Hydrothermal activity lowers trophic diversity in Antarctic sedimented hydrothermal vents[J]. Biogeosciences, 2016,doi: 10.5194/bg-2016-318.
[60] Portail M, Olu K, Dubois S F, et al. Food-web complexity in guaymas basin hydrothermal vents and cold seeps [J]. PLoS One, 2016, 11(9): e0162263. doi: 10.1371/journal.pone.0162263
[61] Vetter R D, Fry B. Sulfur contents and sulfur-isotope compositions of thiotrophic symbioses in bivalve molluscs and vestimentiferan worms [J]. Marine Biology, 1998, 132(3): 453-460. doi: 10.1007/s002270050411
[62] Rodrigues C F, Hilário A, Cunha M R. Chemosymbiotic species from the Gulf of Cadiz (NE Atlantic): distribution, life styles and nutritional patterns [J]. Biogeosciences, 2013, 10(4): 2569-2581. doi: 10.5194/bg-10-2569-2013
[63] Feng D, Cheng M, Kiel S, et al. Using Bathymodiolus tissue stable carbon, nitrogen and sulfur isotopes to infer biogeochemical process at a cold seep in the South China Sea [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2015, 104: 52-59. doi: 10.1016/j.dsr.2015.06.011
[64] Ye F C, Crippa G, Angiolini L, et al. Mapping of recent brachiopod microstructure: a tool for environmental studies [J]. Journal of Structural Biology, 2018, 201(3): 221-236. doi: 10.1016/j.jsb.2017.11.011
[65] Kardon G. Evidence from the fossil record of an antipredatory exaptation: conchiolin layers in corbulid bivalves [J]. Evolution, 1998, 52(1): 68-79. doi: 10.1111/j.1558-5646.1998.tb05139.x
[66] Hein J R, Normark W R, Mcintyre B R, et al. Methanogenic calcite, 13C-depleted bivalve shells, and gas hydrate from a mud volcano offshore southern California [J]. Geology, 2006, 34(2): 109-112. doi: 10.1130/G22098.1
[67] Ambrose W G Jr, Panieri G, Schneider A, et al. Bivalve shell horizons in seafloor pockmarks of the last glacial‐interglacial transition: a thousand years of methane emissions in the Arctic Ocean [J]. Geochemistry, Geophysics, Geosystems, 2015, 16(12): 4108-4129. doi: 10.1002/2015GC005980
[68] Lietard C, Pierre C. Isotopic signatures (δ18O and δ13C) of bivalve shells from cold seeps and hydrothermal vents [J]. Geobios, 2009, 42(2): 209-219. doi: 10.1016/j.geobios.2008.12.001
[69] Dreier A, Loh W, Blumenberg M, et al. The isotopic biosignatures of photo- vs. thiotrophic bivalves: are they preserved in fossil shells? [J]. Geobiology, 2014, 12(5): 406-423. doi: 10.1111/gbi.12093
[70] Kiel S, Taviani M. Chemosymbiotic bivalves from Miocene methane-seep carbonates in Italy [J]. Journal of Paleontology, 2017, 91(3): 444-466. doi: 10.1017/jpa.2016.154
[71] Michener R H, Lajtha K. Stable Isotopes in Ecology and Environmental Science[M]. 2nd ed. Oxford: Blackwell Publishing Ltd, 2007.
[72] O’Donnell T H, Macko S A, Chou J, et al. Analysis of δ13C, δ15N, andδ34S in organic matter from the biominerals of modern and fossil Mercenaria spp. [J]. Organic Geochemistry, 2003, 34(2): 165-183. doi: 10.1016/S0146-6380(02)00160-2
[73] Feng D, Peckmann J, Li N, et al. The stable isotope fingerprint of chemosymbiosis in the shell organic matrix of seep-dwelling bivalves [J]. Chemical Geology, 2018, 479: 241-250. doi: 10.1016/j.chemgeo.2018.01.015
[74] Yan Y X, Sun C J, Huang Y H, et al. Distribution characteristics of lipids in hadal sediment in the Yap Trench [J]. Journal of Oceanology and Limnology, 2020, 38(3): 634-649. doi: 10.1007/s00343-019-8120-2
[75] Ding L, Zhao M X, Yu M, et al. Biomarker assessments of sources and environmental implications of organic matter in sediments from potential cold seep areas of the northeastern South China Sea [J]. Acta Oceanologica Sinica, 2017, 36(10): 8-19. doi: 10.1007/s13131-017-1068-1
[76] Guan H X, Sun Z L, Mao S Y, et al. Authigenic carbonate formation revealed by lipid biomarker inventory at hydrocarbon seeps: a case study from the Okinawa Trough [J]. Marine and Petroleum Geology, 2019, 101: 502-511. doi: 10.1016/j.marpetgeo.2018.12.028
[77] Guerreiro V, Narciso L, Almeida A J, et al. Fatty acid profiles of deep-sea fishes from the Lucky Strike and Menez Gwen hydrothermal vent fields (Mid-Atlantic ridge) [J]. Cybium, 2004, 28(1): 33-44.
[78] Pond D W, Gebruk A, Southward E C, et al. Unusual fatty acid composition of storage lipids in the bresilioid shrimp Rimicaris exoculata couples the photic zone with MAR hydrothermal vent sites [J]. Marine Ecology Progress Series, 2000, 198: 171-179. doi: 10.3354/meps198171
[79] Yamanaka T, Sakata S. Abundance and distribution of fatty acids in hydrothermal vent sediments of the western Pacific Ocean [J]. Organic Geochemistry, 2004, 35(5): 573-582. doi: 10.1016/j.orggeochem.2004.01.002
[80] Li J W, Zhou H Y, Peng X T, et al. Abundance and distribution of fatty acids within the walls of an active deep-sea sulfide chimney [J]. Journal of Sea Research, 2011, 65(3): 333-339. doi: 10.1016/j.seares.2011.01.005
[81] 万志峰, 张伟, 陈崇敏, 等. 琼东南盆地冷泉差异发育特征及其深部控制机理[J]. 海洋地质前沿, 2021, 37(7):1-10
WAN Zhifeng, ZHANG Wei, CHEN Chongmin, et al. Hydrodynamic characteristics of cold seep differential development in the Qiongdongnan Basin and their deep controlling mechanisms [J]. Marine Geology Frontiers, 2021, 37(7): 1-10.
[82] Li J T, Mara P, Schubotz F, et al. Recycling and metabolic flexibility dictate life in the lower oceanic crust [J]. Nature, 2020, 579(7798): 250-255. doi: 10.1038/s41586-020-2075-5
[83] Watkins J M, Hunt J D. A process-based model for non-equilibrium clumped isotope effects in carbonates [J]. Earth and Planetary Science Letters, 2015, 432: 152-165. doi: 10.1016/j.jpgl.2015.09.042
[84] McConnaughey T. 13C and 18O isotopic disequilibrium in biological carbonates: II. In vitro simulation of kinetic isotope effects [J]. Geochimica et Cosmochimica Acta, 1989, 53(1): 163-171. doi: 10.1016/0016-7037(89)90283-4
-



 下载:
下载: