Composition of organic materials and the control factors of suspended particulates in the surface water of the Ross Sea-Amundsen Sea in marginal sea of the southwestern Antarctic in austral summer 2019-2020
-
摘要:
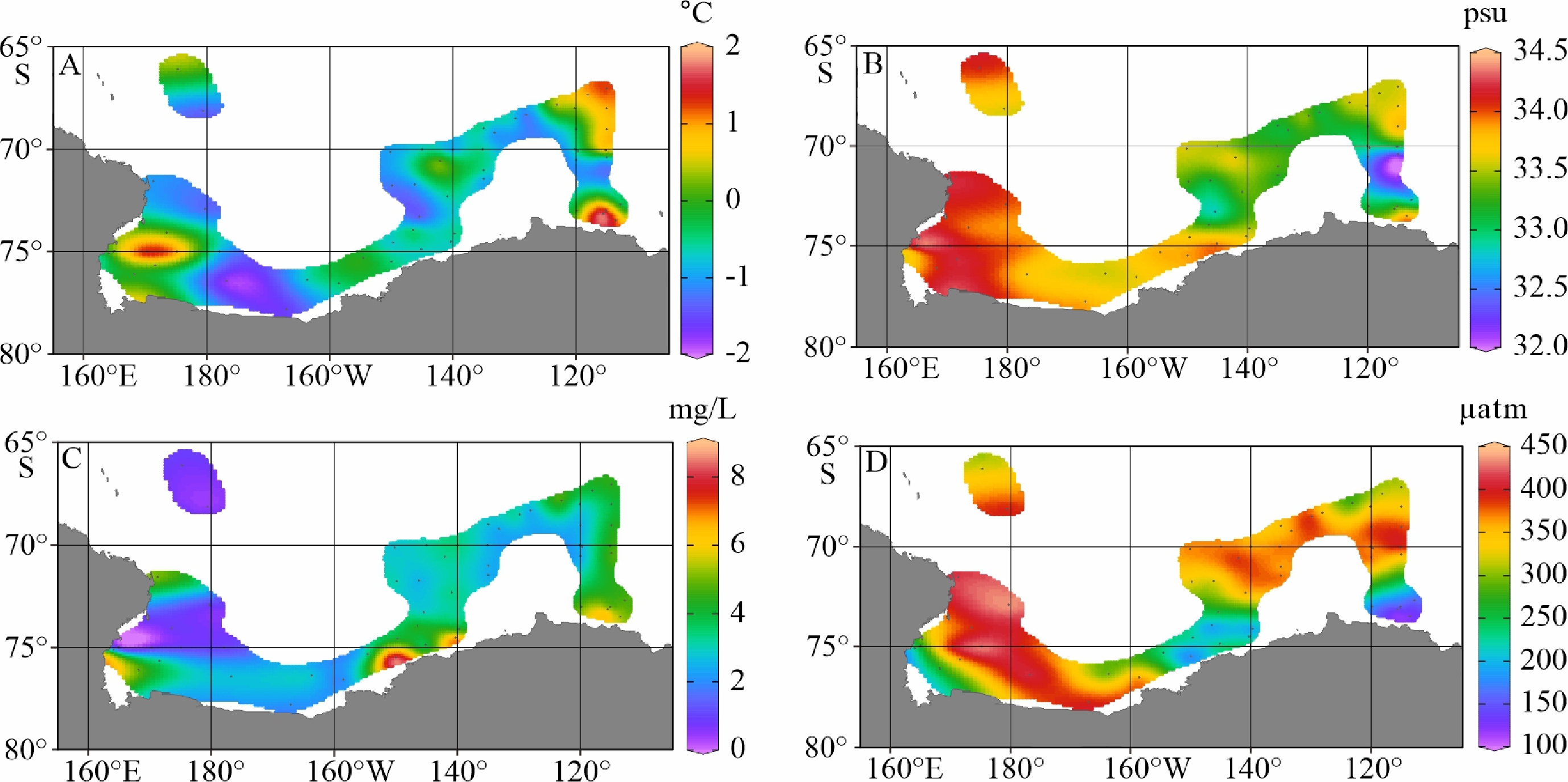
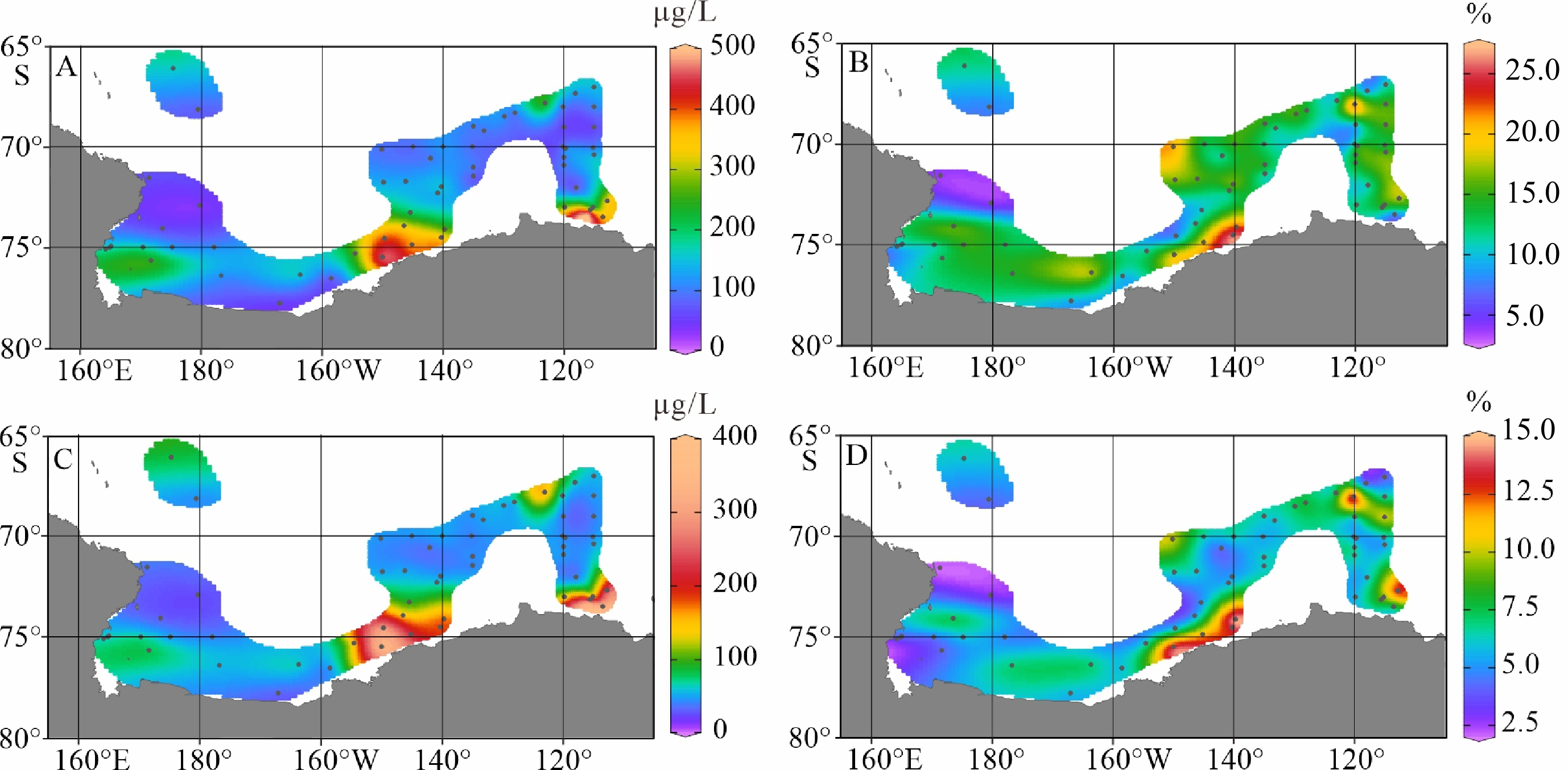
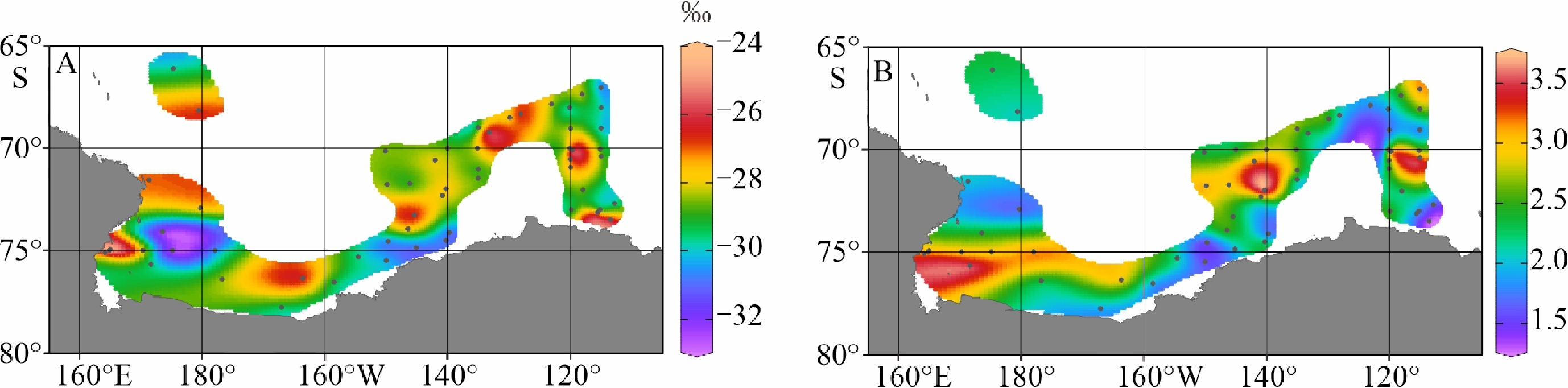
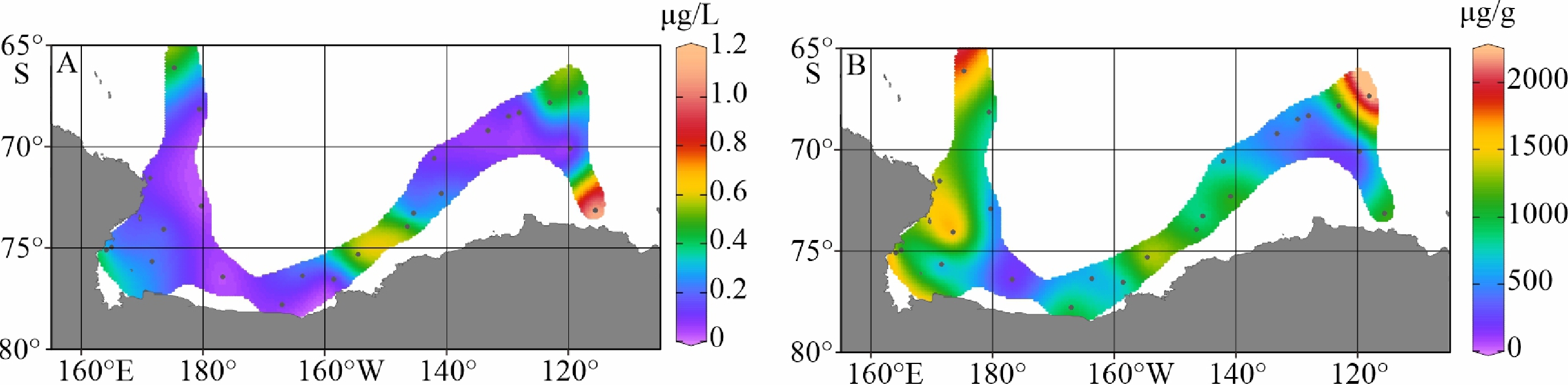
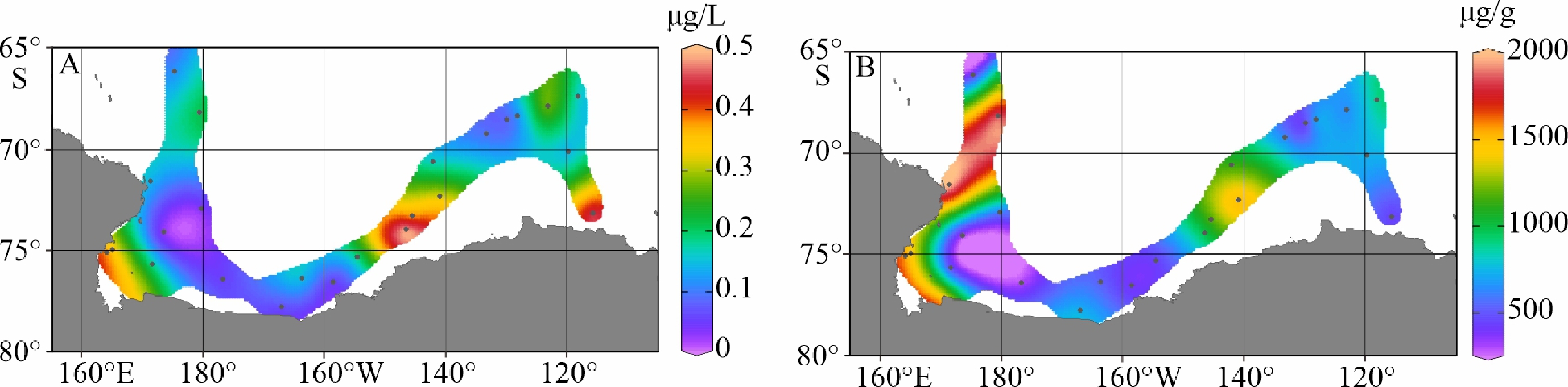
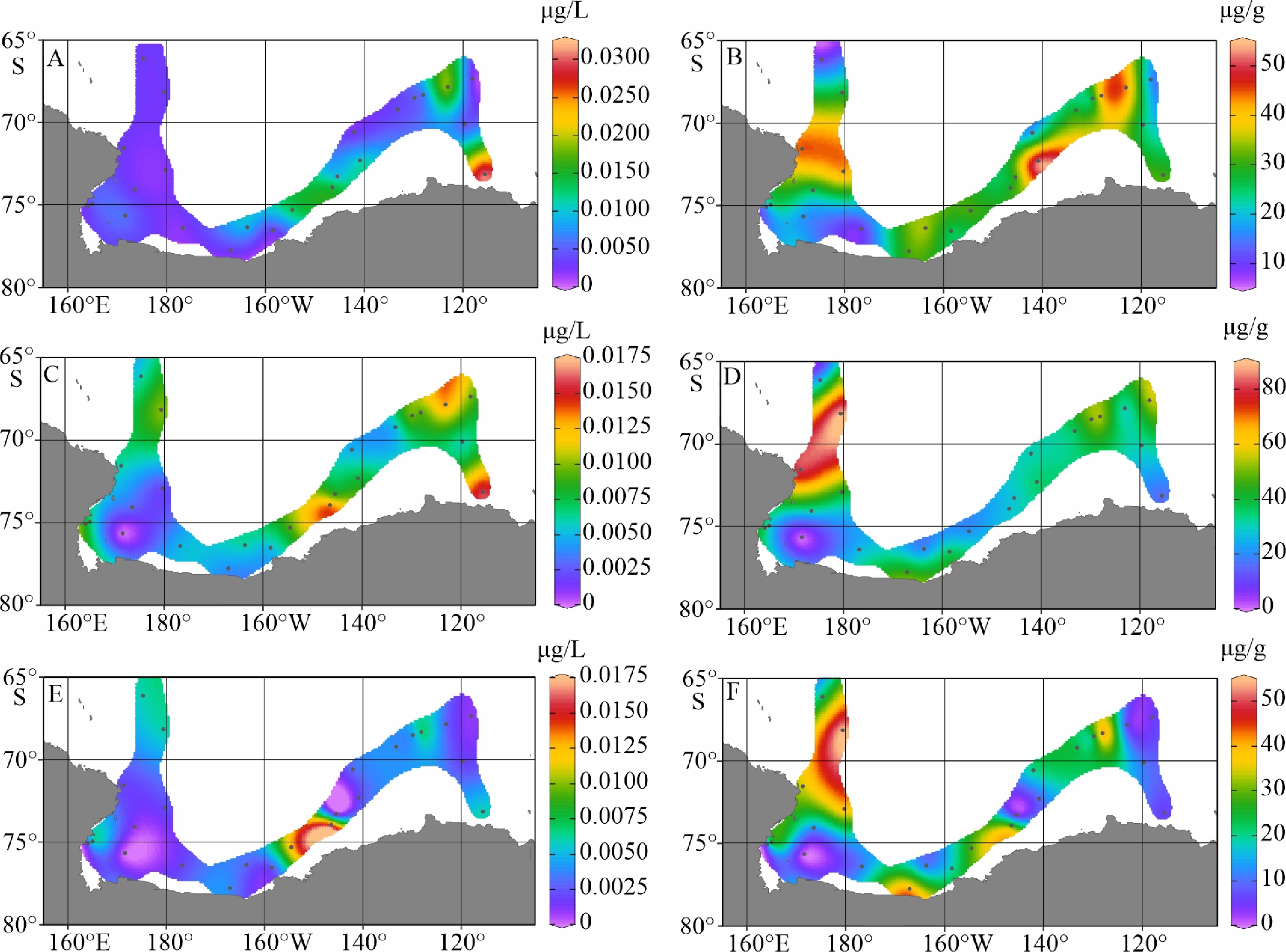
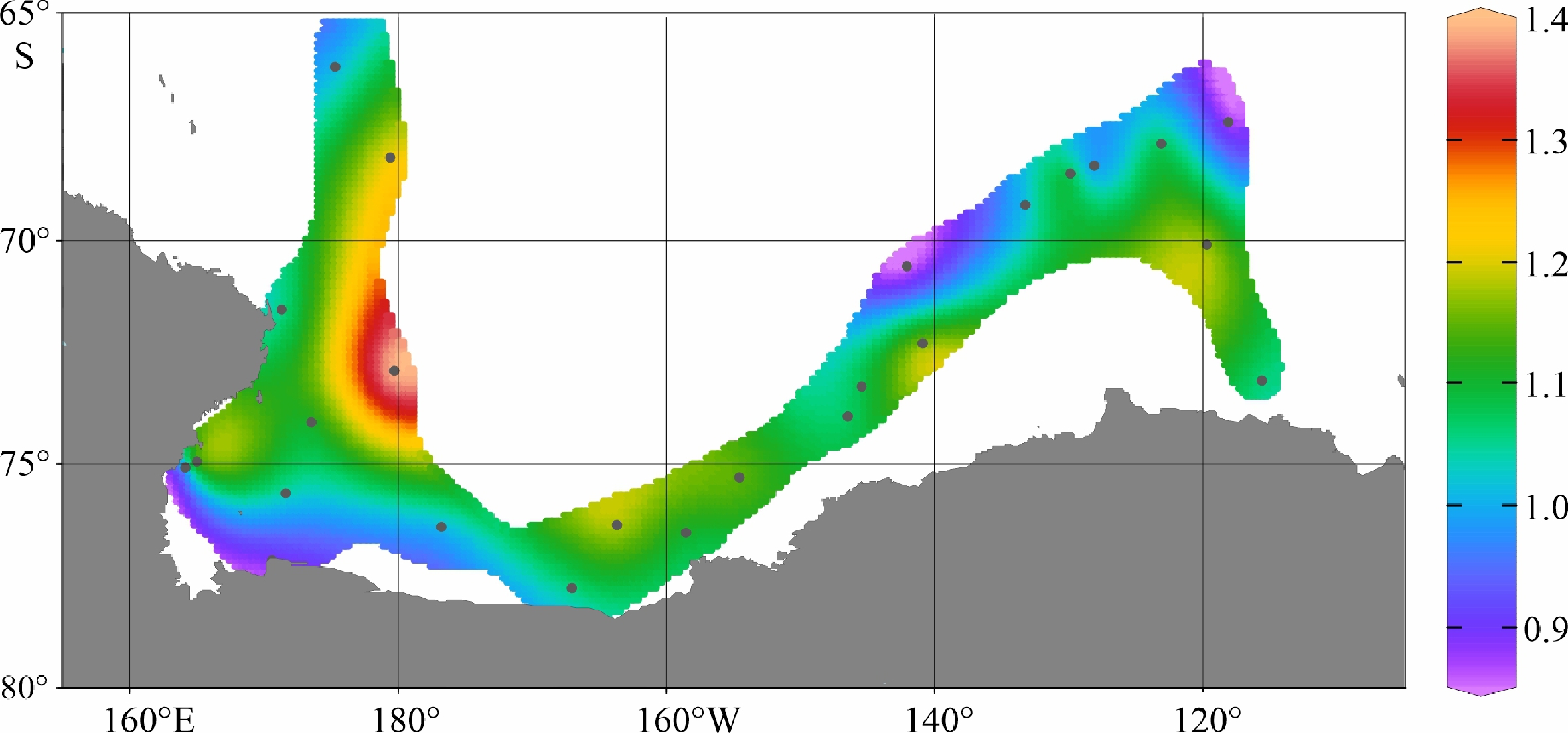
陆架边缘海悬浮颗粒有机质的组成及其来源研究是海洋物质生物地球化学循环研究的重要组成部分。南极的地理位置和气候环境特殊,其边缘海海洋环境受海–气–冰系统共同作用与影响,颗粒有机质的组成及来源具有独特的区域特征与全球意义,其控制因素研究对全面认识罗斯海、阿蒙森海等典型西南极边缘海生物地球化学循环以及生物泵传输效率具有重要的意义。利用中国第36次南极科学考察期间在西南极罗斯海–阿蒙森海区采集的59个表层海水悬浮颗粒物样品的有机碳、氮及其同位素和多种来源特异性的生物标志物分析测试数据,研究了罗斯海–阿蒙森海区域悬浮颗粒物中有机质的物质组成和分布特征,探讨了悬浮颗粒物中不同来源有机质含量与分布的控制因素,评估了不同有机地球化学指标在海–气–冰系统作用下的南极边缘海颗粒有机质组成及来源示踪的应用潜力。研究结果表明,罗斯海–阿蒙森海区海水表层颗粒物中颗粒有机碳(POC)的丰度与表层水体荧光值、海水pCO2、海洋浮游植物源和动物源生物标志物等的丰度分布趋势一致,这种水平分布趋势的一致性表明夏季罗斯海–阿蒙森海海水表层POC主要由浮游植物和动物现场产生。表层悬浮颗粒物总有机质C/N比普遍低于4,显示海区颗粒有机质受微生物降解改造作用明显;表层悬浮颗粒物δ13C值普遍低于−25.2‰,空间分布复杂,反映了南极冰架边缘海独特的贫13C的浮游植物源、富13C的动物源和贫13C的陆源颗粒有机组成的混合信号。表层悬浮颗粒物类脂生物标志物指标是区分不同来源POC组分的有效指标;菜籽甾醇、甲藻甾醇等浮游植物源生物标志物含量之和能较好地反映浮游植物源POC的贡献:近岸冰间湖区呈现高值,其浓度的空间变化受控于水体浮游植物活动;胆甾醇能较好地反映动物源POC的贡献:冰架边缘近岸区呈现高值,其浓度的空间变化受控于海区次级生产力水平和企鹅、海豹等生物量水平;长链的烷基类脂生物标志物较好地反映了南极岩性陆源POC的贡献,其浓度的空间变化受控于南极冰川作用主导的海陆相互作用的过程影响,往往在融冰过程明显的冰架边缘近岸区呈现高值。以上研究结果表明,生物标志物分子指标结合总有机质指标的多参数综合评估为准确辨识南极边缘海复杂的POC来源组成提供了有效的方法,在极区现代海洋(生物地球化学)过程及古海洋环境演化研究中具有广阔的应用空间。
Abstract:The study on the composition and source of suspended particulate organic matter in the marginal sea helps understand marine material biogeochemical cycle. The geographical location and climate environment of Antarctica are special. Antarctic marginal seas are affected by the interaction among marine, atmospheric, and glacial systems. The composition and source of particulate organic carbon (POC) has unique regional characteristics and global significance. Based on the dataset of organic carbon, nitrogen, and their isotopes and source-specific biomarkers extracted from 59 samples of suspended particulate matter collected from surface seawater in the western Antarctic marginal seas during the 36th Antarctic scientific expedition of China, the distribution and composition of organic matter in the suspended particulate matter were studied. The factors controlling the spatial distribution of particulate organic matter were examined, and the application potential of different organic geochemical proxies as the composition and source indicators to particulate organic matter in the Ross Sea-Amundsen Sea was evaluated. Results show that POC concentration in the surface water of the Ross Sea-Amundsen Sea is consistent with the spatial distribution of surface water fluorescence, seawater pCO2, and marine phytoplankton or animal derived biomarkers, which implies that POC in the surface water of Ross Sea-Amundsen Sea in summer is mainly produced by marine phytoplanktons and animals. The C/N ratio is generally below 4, indicating that POC in these regions is obviously degraded by microorganisms. The bulk δ13C value is generally lower than −25.2‰, and the spatial distribution is complex, which reflects the unique mixed POC signals of 13C-depleted phytoplankton source, 13C-enriched animal source , and 13C-depleted terrestrial origins from the Antarctic landscape. Lipid biomarker proxies archived from surface suspended particulate matter is an effective tool to distinguish sources of POC. The concentrations of phytoplankton-derived biomarkers such as brassicasterol and dinosterol reflect the contribution of phytoplankton-derived POC. The nearshore polynya shows a high value, and the spatial variability of its concentration is controlled by the phytoplankton activity in water column. Cholesterol reflects the contribution of animal derived POC. The nearshore at edge of ice shelf shows a high value, and the spatial variability of its concentration is controlled by the secondary productivity in water column and the biomass of penguins and seals. Long chain alkyl lipid biomarkers represent the contribution of lithologic POC from the Antarctic continental region. The spatial variation of their concentration is controlled by glacial activities, and often shows high values in the nearshore area at the edge of ice shelf where obvious ice melting is taken place. This research shows that the comprehensive evaluation of biomarker molecular indicators combined with bulk organic matter indicators provides an effective method for accurately resolving the complex POC matrix in the Antarctic marginal sea.
-
Key words:
- suspended particulates /
- POC /
- biomarkers /
- δ13C /
- material source /
- the Ross Sea-Amundsen Sea
-

-
表 1 主要分析测定目标化合物及其来源信息
Table 1. The target biomarker compounds and the major sources
表 2 罗斯海–阿蒙森海研究区2019−2020年夏季表层水体中悬浮颗粒、总有机碳氮和不同来源生物标志物浓度与海洋环境基本参数空间变化相关性(R)
Table 2. The pearson correlations (R) of concentrations of suspended particulates, POC, PN, and source-specific biomarkers versus marine environmental parameters in the Ross Sea-Amundsen Sea in austral summer of 2019−2020
参数 温度 盐度 荧光叶绿素 海水pCO2 悬浮体浓度 0.427 0.077 0.460 −0.728** 颗粒有机碳(POC)体积浓度 0.293 0.084 0.664** −0.855** 总氮(PN)体积浓度 0.275 0.072 0.663** −0.808** 胆甾醇体积浓度 0.375 −0.172 0.542* −0.769** 浮游植物生物标志物体积浓度 0.744** −0.044 0.564* −0.780** n−C28,30,32脂肪醇体积浓度 0.050 −0.116 0.061 −0.399 n−C26,28,30脂肪酸体积浓度 0.339 −0.474 0.533* −0.615** n−C27,29,31烷烃体积浓度 0.505* −0.336 0.617** −0.662** 注:**代表显著性 p 值为0.01,*代表显著性 p 值为0.05。 表 3 罗斯海–阿蒙森海研究区2019—2020年夏季表层海水多参数空间变化主成分因子分析
Table 3. Principal component analysis of multiple parameters of surface seawater in the Ross Sea-Amundsen Sea in austral summer of 2019−2020 and the loadings of the proxies on factors 1−3
参数 PC1(32.5%) PC2(20.6%) PC3(16.5%) 温度 −0.754 0.370 0.015 盐度 0.077 0.789 0.041 海水pCO2 0.830 0.139 −0.083 总有机质δ13C −0.116 −0.707 0.519 胆甾醇TOC校正浓度 0.072 0.067 0.894 浮游植物生物标志物TOC校正浓度 −0.352 0.751 0.375 n-C28,30,32脂肪醇TOC校正浓度 0.759 0.003 0.299 n-C26,28,30脂肪酸TOC校正浓度 0.650 0.041 0.626 n-C27,29,31烷烃TOC校正浓度 0.327 −0.331 0.310 注:因子PC1—PC3括号内的数字是空间变化总方差的百分比。旋转法:正交Kaiser归一化;某类生物标志物的TOC校正浓度,即某生物标志物占总颗粒有机质的比例。 -
[1] Thomas H, Bozec Y, Elkalay K, et al. Enhanced open ocean storage of CO2 from shelf sea pumping [J]. Science, 2004, 304(5673): 1005-1008. doi: 10.1126/science.1095491
[2] 朱纯, 潘建明, 卢冰, 等. 长江口及邻近海域现代沉积物中正构烷烃分子组合特征及其对有机碳运移分布的指示[J]. 海洋学报, 2005, 27(4):59-67
ZHU Chun, PAN Jianming, LU Bing, et al. Compositional feature of n-alkanes in modern sediment from the Changjiang Estuary and adjacent area and its implication to transport and distribution of organic carbon [J]. Acta Oceanologica Sinica, 2005, 27(4): 59-67.
[3] 高建华, 汪亚平, 潘少明, 等. 长江口外海域沉积物中有机物的来源及分布[J]. 地理学报, 2007, 62(9):981-991 doi: 10.3321/j.issn:0375-5444.2007.09.009
GAO Jianhua, WANG Yaping, PAN Shaoming, et al. Source and distribution of organic matter in seabed sediments of the Changjiang River estuary and its adjacent sea area [J]. Acta Geographica Sinica, 2007, 62(9): 981-991. doi: 10.3321/j.issn:0375-5444.2007.09.009
[4] Liu K K, Atkinson L, Quiñones R, et al. Carbon and Nutrient Fluxes in Continental Margins: A Global Synthesis[M]. Berlin: Springer, 2010: 741.
[5] Blair N E, Aller R C. The fate of terrestrial organic carbon in the marine environment [J]. Annual Review of Marine Science, 2012, 4: 401-423. doi: 10.1146/annurev-marine-120709-142717
[6] 胡静文, 张海龙, 李莉, 等. 长江口悬浮颗粒物中有机质来源及厌氧氨氧化活动的季节性变化[J]. 中国科学:地球科学, 2016, 59(7):1339-1352 doi: 10.1007/s11430-016-5286-8
HU Jingwen, ZHANG Hailong, LI Li, et al. Seasonal changes of organic matter origins and anammox activity in the Changjiang Estuary deduced from multi-biomarkers in suspended particulates [J]. Science China Earth Sciences, 2016, 59(7): 1339-1352. doi: 10.1007/s11430-016-5286-8
[7] Keil R. Anthropogenic forcing of carbonate and organic carbon preservation in marine sediments [J]. Annual Review of Marine Science, 2017, 9: 151-172. doi: 10.1146/annurev-marine-010816-060724
[8] 焦念志, 梁彦韬, 张永雨, 等. 中国海及邻近区域碳库与通量综合分析[J]. 中国科学:地球科学, 2018, 61(11):1535-1563 doi: 10.1007/s11430-018-9190-x
JIAO Nianzhi, LIANG Yantao, ZHANG Yongyu, et al. Carbon pools and fluxes in the China Seas and adjacent oceans [J]. Science China Earth Sciences, 2018, 61(11): 1535-1563. doi: 10.1007/s11430-018-9190-x
[9] Arrigo K R, Worthen D, Schnell A, et al. Primary production in Southern Ocean waters [J]. Journal of Geophysical Research:Oceans, 1998, 103(C8): 15587-15600. doi: 10.1029/98JC00930
[10] Stammerjohn S, Massom R, Rind D, et al. Regions of rapid sea ice change: an inter-hemispheric seasonal comparison [J]. Geophysical Research Letters, 2012, 39(6): L06501.
[11] Wu Y, Zhang J, Mi T Z, et al. Occurrence of n-alkanes and polycyclic aromatic hydrocarbons in the core sediments of the Yellow Sea [J]. Marine Chemistry, 2001, 76(1-2): 1-15. doi: 10.1016/S0304-4203(01)00040-8
[12] 黄清辉, 沈涣庭. 河口海岸环境中有机污染标志物的研究意义[J]. 海洋科学, 2001, 25(12):18-20 doi: 10.3969/j.issn.1000-3096.2001.12.005
HUANG Qinghui, SHEN Huanting. Significance of molecule markers in study the pollution in the estuarine and coastal environments [J]. Marine Sciences, 2001, 25(12): 18-20. doi: 10.3969/j.issn.1000-3096.2001.12.005
[13] Hopmans E C, Weijers J W H, Schefuß E, et al. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids [J]. Earth and Planetary Science Letters, 2004, 224(1-2): 107-116. doi: 10.1016/j.jpgl.2004.05.012
[14] Li X X, Bianchi T S, Allison M A, et al. Composition, abundance and age of total organic carbon in surface sediments from the inner shelf of the East China Sea [J]. Marine Chemistry, 2012, 145-147: 37-52. doi: 10.1016/j.marchem.2012.10.001
[15] Liu B, He Y X, Zhang Y Z, et al. Natural and anthropogenic organic matter cycling between coastal wetlands and rivers: a case study from Liao River Delta [J]. Estuarine, Coastal and Shelf Science, 2020, 236: 106610. doi: 10.1016/j.ecss.2020.106610
[16] Feng X J, Vonk J E, Van Dongen B E, et al. Differential mobilization of terrestrial carbon pools in Eurasian Arctic river basins [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(35): 14168-14173. doi: 10.1073/pnas.1307031110
[17] Collister J W, Rieley G, Stern B, et al. Compound-specific δ13C analyses of leaf lipids from plants with differing carbon dioxide metabolisms [J]. Organic Geochemistry, 1994, 21(6-7): 619-627. doi: 10.1016/0146-6380(94)90008-6
[18] Schubert C J, Calvert S E. Nitrogen and carbon isotopic composition of marine and terrestrial organic matter in Arctic Ocean sediments: : implications for nutrient utilization and organic matter composition [J]. Deep Sea Research Part I:Oceanographic Research Papers, 2001, 48(3): 789-810. doi: 10.1016/S0967-0637(00)00069-8
[19] Blair N, Leu A, Muñoz E, et al. Carbon isotopic fractionation in heterotrophic microbial metabolism [J]. Applied and Environmental Microbiology, 1985, 50(4): 996-1001. doi: 10.1128/aem.50.4.996-1001.1985
[20] Keil R G, Tsamakis E, Fuh C B, et al. Mineralogical and textural controls on the organic composition of coastal marine sediments: Hydrodynamic separation using SPLITT-fractionation [J]. Geochimica et Cosmochimica Acta, 1994, 58(2): 879-893. doi: 10.1016/0016-7037(94)90512-6
[21] Ehleringer J R, Buchmann N, Flanagan L B. Carbon isotope ratios in belowground carbon cycle processes [J]. Ecological Applications, 2000, 10(2): 412-422. doi: 10.1890/1051-0761(2000)010[0412:CIRIBC]2.0.CO;2
[22] Waterson E J, Canuel E A. Sources of sedimentary organic matter in the Mississippi River and adjacent Gulf of Mexico as revealed by lipid biomarker and δ13CTOC analyses [J]. Organic Geochemistry, 2008, 39(4): 422-439. doi: 10.1016/j.orggeochem.2008.01.011
[23] Schreiner K M, Blair N E, Levinson W, et al. Contribution of fungal macromolecules to soil carbon sequestration[M]//Hartemink A, McSweeney K. Soil Carbon. Cham: Springer, 2014: 155-161.
[24] Meyers P A. Preservation of elemental and isotopic source identification of sedimentary organic matter [J]. Chemical Geology, 1994, 114(3-4): 289-302. doi: 10.1016/0009-2541(94)90059-0
[25] Lee S, Fuhrman J A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton [J]. Applied and Environmental Microbiology, 1987, 53(6): 1298-1303. doi: 10.1128/aem.53.6.1298-1303.1987
[26] Coffin R B, Cifuentes L A, Approaches for measuring stable carbon and nitrogen isotopes in bacteria[M]//Kemp P F, Sherr B F, Sherr E B, et al. Handbook of Methods in Aquatic Microbial Ecology. Boca Raton: CRC Press, 1993.
[27] 尹希杰, 李云海, 乔磊, 等. 南极普里兹湾海域夏季表层水体颗粒有机碳及其同位素分布特征[J]. 极地研究, 2014, 26(1): 159-166.
YIN Xijie, LI Yunhai, QIAO Lei, et al. Distribution of particulate organic carbon (POC) and δ13CPOC in surface waters in summer in Prydz Bay, Antarctica[J]. Chinese Journal of Polar Research, 2014, 26(1): 159-166.
[28] 韩正兵, 扈传昱, 薛斌, 等. 2007/2008年和2008/2009年夏季南大洋以及普里兹湾POC的分布与变化[J]. 极地研究, 2011, 23(1):11-18
HAN Zhengbing, HU Chuanyu, XUE Bin, et al. Particulate organic carbon in the surface water of south ocean and prydz bay during the austral summer of 2007/2008 and 2008/2009 [J]. Chinese Journal of Polar Research, 2011, 23(1): 11-18.
[29] Fry B, Sherr E B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems[M]//Rundel P W, Ehleringer J R, Nagy K A. Stable Isotopes in Ecological Research. New York: Springer, 1989: 196-229.
[30] Hedges J I, Keil R G, Benner R. What happens to terrestrial organic matter in the ocean? [J]. Organic Geochemistry, 1997, 27(5-6): 195-212. doi: 10.1016/S0146-6380(97)00066-1
[31] Aller R C, Blair N E. Carbon remineralization in the Amazon-Guianas tropical mobile mudbelt: a sedimentary incinerator [J]. Continental Shelf Research, 2006, 26(17-18): 2241-2259. doi: 10.1016/j.csr.2006.07.016
[32] Wiesenberg G L B, Schwarzbauer J, Schmidt M W I, et al. Source and turnover of organic matter in agricultural soils derived from n-alkane/n-carboxylic acid compositions and C-isotope signatures [J]. Organic Geochemistry, 2004, 35(11-12): 1371-1393. doi: 10.1016/S0146-6380(04)00122-6
[33] Weijers J W H, Schouten S, Schefuß E, et al. Disentangling marine, soil and plant organic carbon contributions to continental margin sediments: a multi-proxy approach in a 20, 000 year sediment record from the Congo deep-sea fan [J]. Geochimica et Cosmochimica Acta, 2009, 73(1): 119-132. doi: 10.1016/j.gca.2008.10.016
[34] Kopczyńska E E, Goeyens L, Semeneh M, et al. Phytoplankton composition and cell carbon distribution in Prydz Bay, Antarctica: relation to organic particulate matter and its δ13C values [J]. Journal of Plankton Research, 1995, 17(4): 685-707. doi: 10.1093/plankt/17.4.685
[35] Cozzi S, Cantoni C. Stable isotope (δ13C and δ15N) composition of particulate organic matter, nutrients and dissolved organic matter during spring ice retreat at Terra Nova Bay [J]. Antarctic Science, 2011, 23(1): 43-56. doi: 10.1017/S0954102010000611
[36] 于培松, 张海生, 扈传昱, 等. 利用沉积生物标志物分析南极普里兹湾浮游植物群落结构变化[J]. 极地研究, 2012, 24(2):143-150
YU Peisong, ZHANG Haisheng, HU Chuanyu, et al. Using biomarkers in sediments as indicators to rebuild the phytoplankton community in Prydz Bay, Antarctica [J]. Chinese Journal of Polar Research, 2012, 24(2): 143-150.
[37] Petty A A, Feltham D L, Holland P R. Impact of atmospheric forcing on antarctic continental shelf water masses [J]. Journal of Physical Oceanography, 2013, 43(5): 920-940. doi: 10.1175/JPO-D-12-0172.1
[38] Sweeney C. The annual cycle of surface water CO2 and O2 in the Ross Sea: a model for gas exchange on the continental shelves of Antarctica[M]//Ditullio G R, Dunbar R B. Biogeochemistry of the Ross Sea. American Geophysical Union, 2003: 295-312.
[39] Lee S, Hwang J, Ducklow H W, et al. Evidence of minimal carbon sequestration in the productive Amundsen Sea polynya [J]. Geophysical Research Letters, 2017, 44(15): 7892-7899. doi: 10.1002/2017GL074646
[40] Collier R, Dymond J, Honjo S, et al. The vertical flux of biogenic and lithogenic material in the Ross Sea: moored sediment trap observations 1996-1998 [J]. Deep Sea Research Part II:Topical Studies in Oceanography, 2000, 47(15-16): 3491-3520. doi: 10.1016/S0967-0645(00)00076-X
[41] 杨和福. 棕囊藻的生物学概述: I. 形态分类和生理生态学[J]. 东海海洋, 2004, 22(1):49-63
YANG Hefu. The biology of Phaeocystis: I. the morphology, physiology and ecology of Phaeocystis [J]. Donghai Marine Science, 2004, 22(1): 49-63.
[42] Jacobs S S. On the nature and significance of the Antarctic Slope Front [J]. Marine Chemistry, 1991, 35(1-4): 9-24. doi: 10.1016/S0304-4203(09)90005-6
[43] Tao S Q, Eglinton T I, Montluçon D B, et al. Pre-aged soil organic carbon as a major component of the Yellow River suspended load: Regional significance and global relevance [J]. Earth and Planetary Science Letters, 2015, 414: 77-86. doi: 10.1016/j.jpgl.2015.01.004
[44] 丁玲, 邢磊, 赵美训. 生物标志物重建浮游植物生产力及群落结构研究进展[J]. 地球科学进展, 2010, 25(9):981-989
DING Ling, XING Lei, ZHAO Meixun. Applications of biomarkers for reconstructing phytoplankton productivity and community structure changes [J]. Advances in Earth Science, 2010, 25(9): 981-989.
[45] Volkman J K, Barrett S M, Blackburn S I, et al. Microalgal biomarkers: a review of recent research developments [J]. Organic Geochemistry, 1998, 29(5-7): 1163-1179. doi: 10.1016/S0146-6380(98)00062-X
[46] Boon J J, Rijpstra W I C, De Lange F, et al. Black Sea sterol— a molecular fossil for dinoflagellate blooms [J]. Nature, 1979, 277(5692): 125-127. doi: 10.1038/277125a0
[47] Tao S Q, Xing L, Luo X F, et al. Alkenone distribution in surface sediments of the southern Yellow Sea and implications for the UKʹ 37 thermometer [J]. Geo-Marine Letters, 2012, 32(1): 61-71. doi: 10.1007/s00367-011-0251-1
[48] Otto A, Simpson M J. Degradation and preservation of vascular plant-derived biomarkers in grassland and forest soils from western Canada [J]. Biogeochemistry, 2005, 74(3): 377-409. doi: 10.1007/s10533-004-5834-8
[49] Zhao M X, Mercer J L, Eglinton G, et al. Comparative molecular biomarker assessment of phytoplankton paleoproductivity for the last 160 kyr off Cap Blanc, NW Africa [J]. Organic Geochemistry, 2006, 37(1): 72-97. doi: 10.1016/j.orggeochem.2005.08.022
[50] Eglinton G, Hamilton R J. Leaf epicuticular waxes: the waxy outer surfaces of most plants display a wide diversity of fine structure and chemical constituents [J]. Science, 1967, 156(3780): 1322-1335. doi: 10.1126/science.156.3780.1322
[51] Pearson A, Eglinton T I. The origin of n-alkanes in Santa Monica Basin surface sediment: a model based on compound-specific Δ14C and δ13C data [J]. Organic Geochemistry, 2000, 31(11): 1103-1116. doi: 10.1016/S0146-6380(00)00121-2
[52] Collister J W, Lichtfouse E, Hieshima G, et al. Partial resolution of sources of n-alkanes in the saline portion of the Parachute Creek Member, Green River Formation (Piceance Creek Basin, Colorado) [J]. Organic Geochemistry, 1994, 21(6-7): 645-659. doi: 10.1016/0146-6380(94)90010-8
[53] Arrigo K R, Van Dijken G L. Phytoplankton dynamics within 37 Antarctic coastal polynya systems [J]. Journal of Geophysical Resea-rch:Oceans, 2003, 108(C8): 3271. doi: 10.1029/2002JC001739
[54] Arrigo K R, Lowry K E, Van Dijken G L. Annual changes in sea ice and phytoplankton in polynyas of the Amundsen Sea, Antarctica [J]. Deep Sea Research II:Topical Studies in Oceanography, 2012, 71-76: 5-15. doi: 10.1016/j.dsr2.2012.03.006
[55] Popp B N, Trull T, Kenig F, et al. Controls on the carbon isotopic composition of Southern Ocean phytoplankton [J]. Global Biogeochemical Cycles, 1999, 13(4): 827-843. doi: 10.1029/1999GB900041
[56] Damsté J S S, Schouten S, Hopmans E C, et al. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota [J]. Journal of Lipid Research, 2002, 43(10): 1641-1651. doi: 10.1194/jlr.M200148-JLR200
[57] 杨和福. 棕囊藻的生物学概述: Ⅱ. 生理生化学[J]. 东海海洋, 2004, 22(3):34-47
YANG Hefu. The biology of Phaeocystis: Ⅱ. The physiology and biochemistry of Phaeocystis [J]. Donghai Marine Science, 2004, 22(3): 34-47.
[58] 邢磊, 赵美训, 张海龙, 等. 二百年来黄海浮游植物群落结构变化的生物标志物记录[J]. 中国海洋大学学报:自然科学版, 2009, 39(2):317-322
XING Lei, ZHAO Meixun, ZHANG Hailong, et al. Biomarker records of phytoplankton community structure changes in the Yellow Sea over the last 200 years [J]. Periodical of Ocean University of China, 2009, 39(2): 317-322.
[59] Goñi M A, Ruttenberg K C, Eglinton T I. Sources and contribution of terrigenous organic carbon to surface sediments in the Gulf of Mexico [J]. Nature, 1997, 389(6648): 275-278. doi: 10.1038/38477
[60] Goñi M A, Montgomery S. Alkaline CuO oxidation with a microwave digestion system: lignin analyses of geochemical samples [J]. Analytical Chemistry, 2000, 72(14): 3116-3121. doi: 10.1021/ac991316w
[61] Goñi M A, Cathey M W, Kim Y H, et al. Fluxes and sources of suspended organic matter in an estuarine turbidity maximum region during low discharge conditions [J]. Estuarine, Coastal and Shelf Science, 2005, 63(4): 683-700. doi: 10.1016/j.ecss.2005.01.012
-




 下载:
下载: