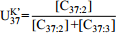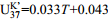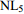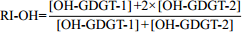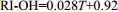Progresses in the study of organic lipid molecules for reconstruction of paleo-sea temperature
-
摘要:
温度是气候变化中一个非常敏感和关键的因子,也是气候模拟试验中不可或缺的边界条件。古温度重建对于理解古气候系统(如大气环流、洋流强度和路径演化历史)及预测未来气候变化都具有重要意义。随着分析测试技术的不断发展,分子有机地球化学温度指标受到高度重视,成为古气候研究的重要手段,迄今为止,已在全球范围内得到了广泛应用。本文综述了
${\rm U}^{\rm K}_{37} $ Abstract:Temperature is a very sensitive and crucial factor in climate change, and an indispensable boundary condition in climate modelling. The reconstruction of paleotemperature is of great significance for understanding paleoclimate system such as the evolution of atmospheric circulation, and ocean currents strength and path, as well as for making more accurate predictions of future climate changes. Along with the development of new analytical techniques, organic temperature proxies have been highly valued as an important tool in paleoclimate research and widely applied in temperature reconstruction globally. In this paper, six organic thermometers are reviewed, i.e.
${\rm U}^{\rm K}_{37} $ -
Key words:
- paleotemperature reconstruction /
- organic proxy /
- lipids /
- marine environment
-

-
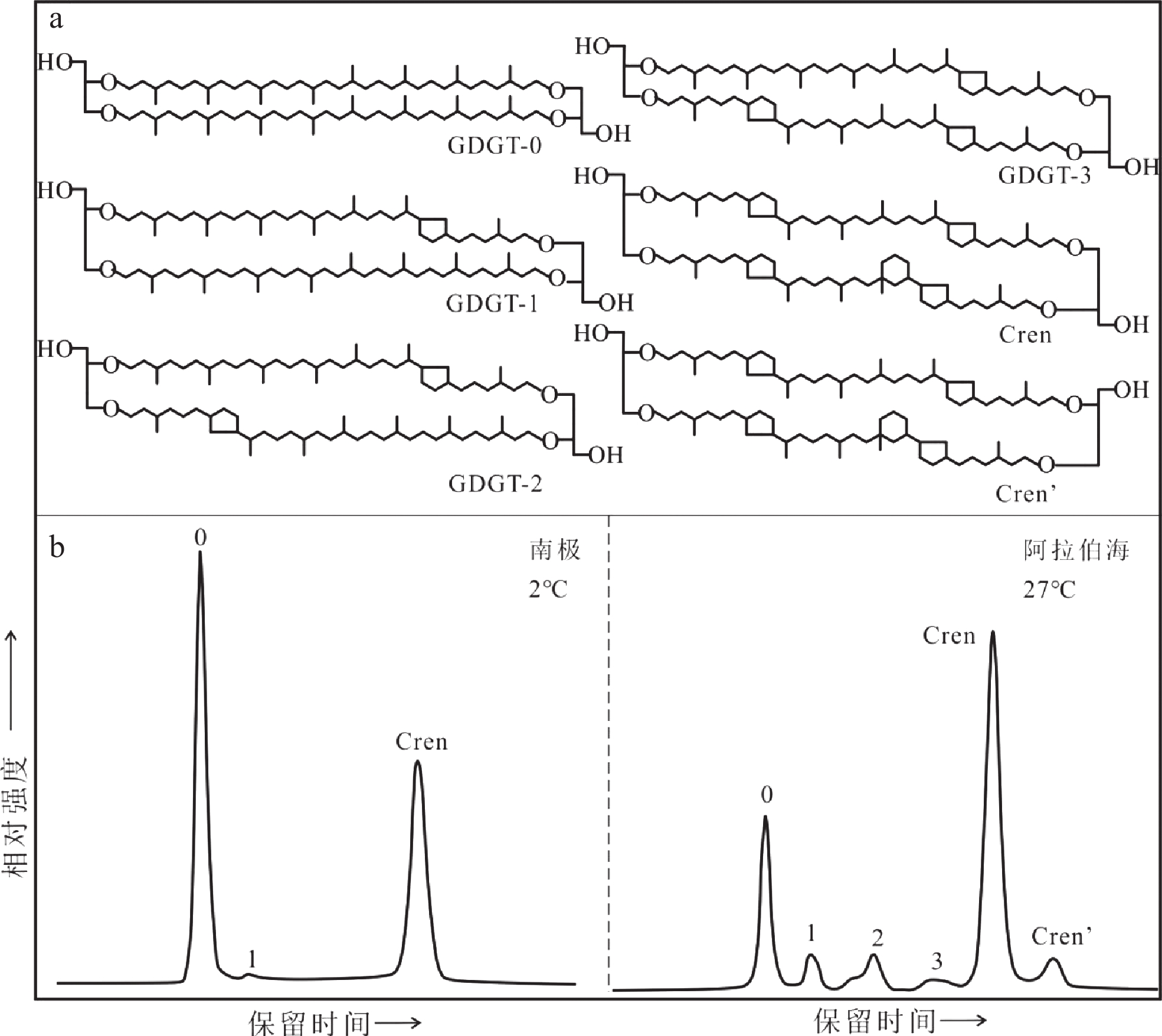
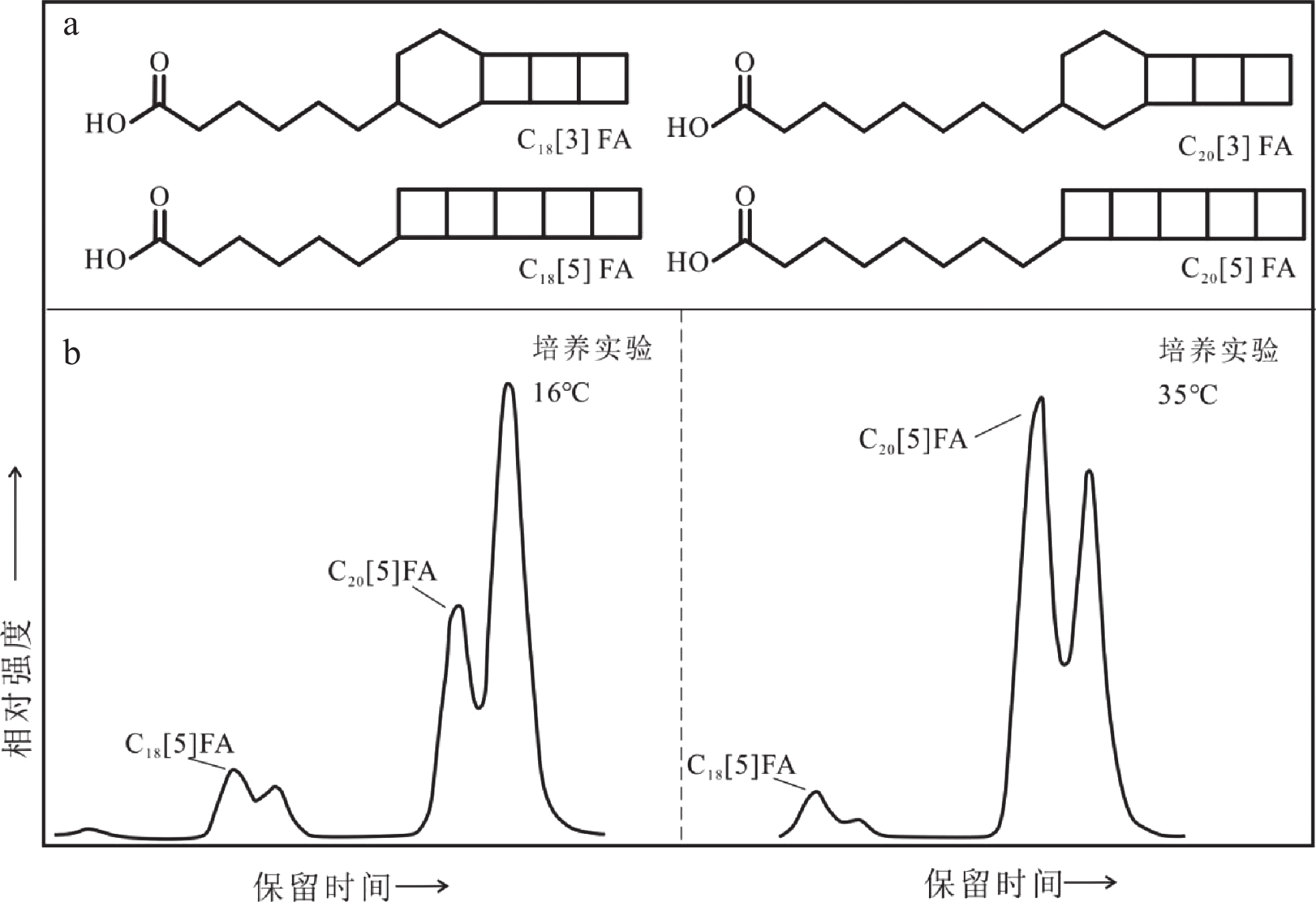
图 3 梯烷脂脂肪酸分子结构(a,FA: fatty acid)和不同温度下Candidatus B. fulgida培养实验中梯烷脂脂肪酸的代表性色谱图(b)[65]
Figure 3.
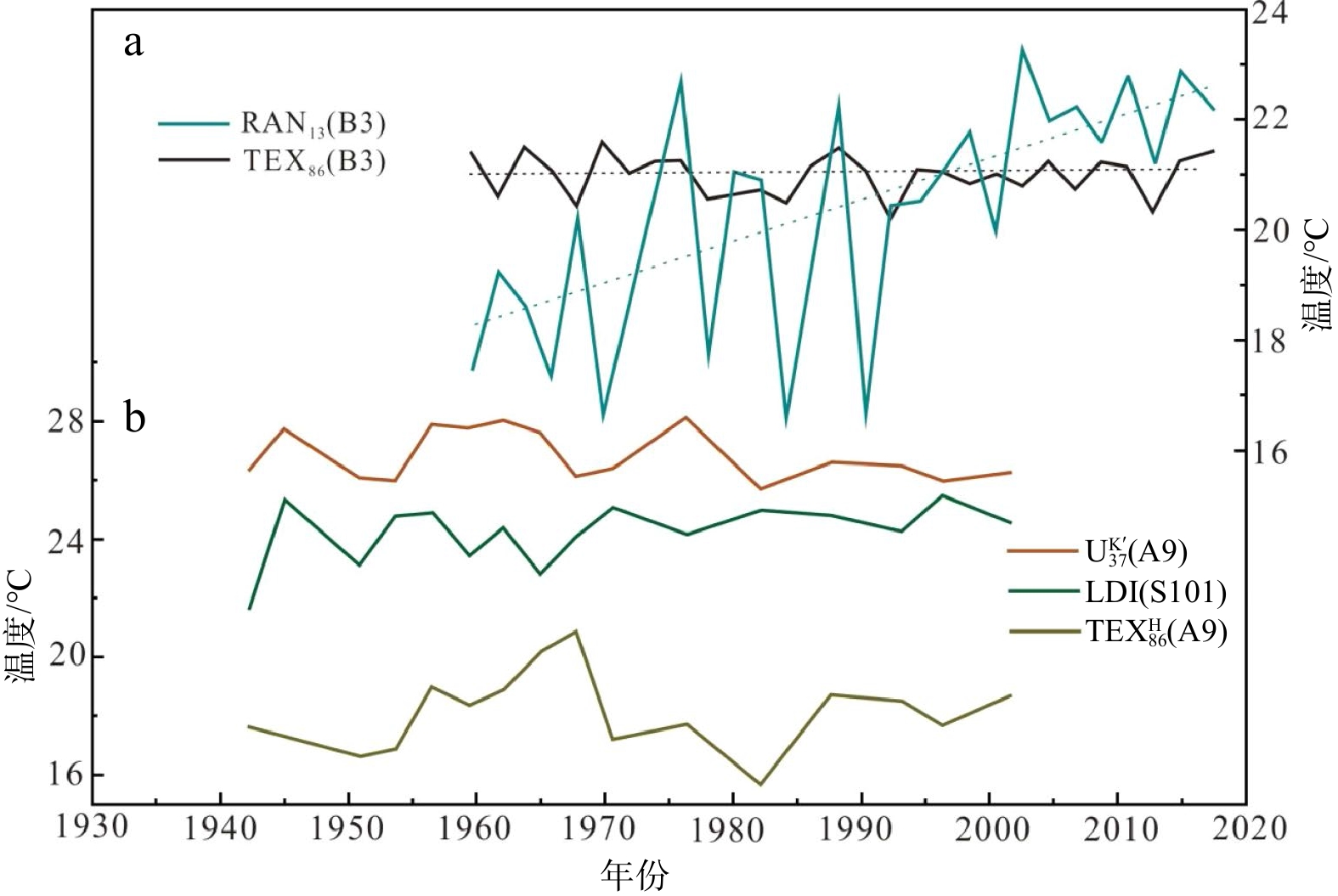
表 1 长链烯酮古温度指标(
${\rm U}^{\rm K}_{37} $ )及温度校准公式Table 1. Paleotemperature
${\rm U}^{\rm K}_{37} $ index (derived from long-chain alkenones) and the temperature calibration equations表 2 四醚膜脂古温度指标(TEX86)及温度校准公式
Table 2. Paleotemperature TEX86 index (derived from iGDGTs) and the temperature calibration equations
TEX86指标 序号 公式 指示意义 参考文献 1 
海水温度 [43] 2 
[44] 3 
[48] 
4 
陆源和海源有机质的相对丰度 [49] 温度校准公式 样品来源 公式 适用范围 样品数 r2 标准误差 参考文献 5 培养实验 
5~35℃ 15 0.79 − [36] 6 北冰洋表层沉积物 
0~30℃ 104 0.93 − [44] 7 全球大洋表层沉积物(无红海,无极地) 
5~30℃ 223 0.935 1.7 [45] 8 红海北部表层沉积物 
24.6~28.8℃ 11 0.90 0.36 [46] 9 全球大洋表层沉积物 
0~30℃ 44 0.92 2.0 [43] 10 
−2~30℃ 287 0.817 3.7 [47] 11 
−3~30℃ 396 0.86 4.0 [48] 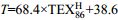
5~30℃ 255 0.87 2.5 注:Ia、IIa(IIa')、IIIa(IIIa')属于bGDGTs。 表 3 梯烷脂古温度指标(NL5)及温度校准公式
Table 3. Paleotemperature NL5 (ladderane lipids) index and the tempreature calibrations equations
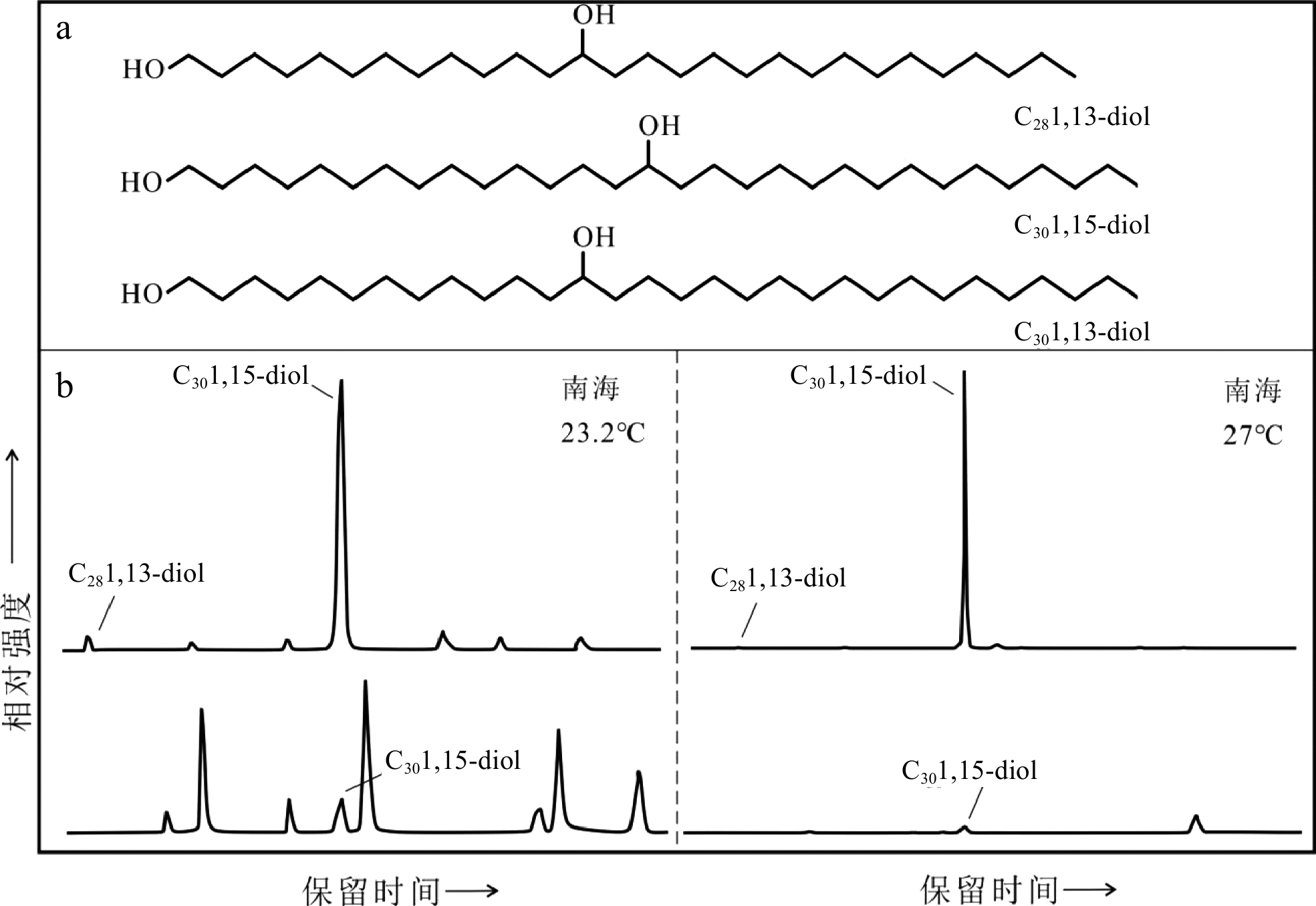
表 4 长链烷基二醇古温度指标(LDI)及温度校准公式
Table 4. Paleotemperature LDI (long chain diol index) and the calibration equations
表 5 羟基四醚膜脂古温度指标(RI-OH)及温度校准公式
Table 5. Paleotemperature RI-OH indices derived from OH-GDGTs and the tempreature calibration equations
表 6 3-羟基脂肪酸指标及温度、pH校准公式
Table 6. The 3-OH-FAs index, and paleotemperature and pH calibration equations
3-羟基脂肪酸指标 序号 指标 公式 指示意义 参考文献 1 支链比 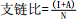
pH [114] 2 

3 支链指数 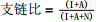
4 RIN 
5 
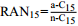
6 
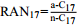
大气年平均温度 [114] 7 

[119] 8 

海水表层温度 [120] 温度校准公式 样品来源 公式 适用范围 样品数 r2 标准误差 参考文献 9 神农架土壤 
4.49~7.98 26 0.76 − [114] 10 
0.70 0.54 11 
0.70 0.54 12 
0.67 0.56 13 MAT=23.03–3.03×RAN15 1.9~14.7℃ 0.51 2.6 14 MAT=26.36–9.09×RAN17 0.48 2.7 15 Majella山土壤 MAT=13.54–1.94×RAN15 0.2~14.1℃ 11 0.52 2.9 [124] 16 Rungwe山土壤 MAT=30.50–3.19×RAN15 14.3~25.7℃ 28 0.52 1.6 17 神农架、Majella山、Rungwe山土壤 MAT=25.74–7.38×RAN17 0.2~25.7℃ 65 0.60 5.1 18 北太平洋表层沉积物 
1.3~28.1℃ 45 0.92 2.55 [120] 19 中国碱性淡水湖 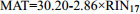
4.9~17℃ 24 0.65 2.6 [119] 注:N/n代表正构,I/i代表异构,A/a代表反异构。 表 7 不同脂质古温度指标综合对比
Table 7. Comparison among different lipid-based temperature proxies
指标 生物来源 适用范围 关键影响因素 温度 年代 典型区域 
定鞭藻 −2~29℃ 新近纪至今  :中低纬深海海域
:中低纬深海海域 :高纬深海海域
:高纬深海海域繁殖季节、侧向输入 TEX86 古菌 <38.6℃ 侏罗纪至今  :<15℃海域
:<15℃海域 :>15℃海域
:>15℃海域
不适用于甲烷活动海域陆源输入、繁殖季节和水深、成熟度、甲烷活动 NL5 厌氧氨氧化菌 12~20℃ 第四纪至今 近岸沿海海域 营养盐、溶解有机碳浓度、水深、氧含量、早期成岩作用 LDI 硅藻Proboscia、
异鞭藻A. radians、真眼点藻−3~30℃ 新近纪至今 − 淡水输入、氧化降解 RI-OH 古菌 <29℃ 第四纪至今 RI-OH:>15℃海域
RI-OH':<15℃海域陆源输入、繁殖季节 RAN13 革兰氏阴性菌 >0℃ − − − 注:适用范围来自现有资料,未来可能有更广阔的适用性。 -
[1] Elderfield H E, Ganssen G M. Past temperature and δ18O of surface ocean waters inferred from foraminiferal Mg/Ca ratios [J]. Nature, 2000, 405(6785): 442-445. doi: 10.1038/35013033
[2] Erez J, Luz B. Experimental paleotemperature equation for planktonic foraminifera [J]. Geochimica et Cosmochimica Acta, 1983, 47(6): 1025-1031. doi: 10.1016/0016-7037(83)90232-6
[3] Spero H J, Bijma J, Lea D W, et al. Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes [J]. Nature, 1997, 390(6659): 497-500. doi: 10.1038/37333
[4] Eglinton T I, Eglinton G. Molecular proxies for paleoclimatology[J]. Earth and Planetary Science Letters, 2008, 275(1–2): 1–16.
[5] Rechka J A, Maxwell J R. Characterisation of alkenone temperature indicators in sediments and organisms[J]. Organic Geochemistry, 1988, 13(4–6): 727–734.
[6] Prahl F G, Wakeham S G. Calibration of unsaturation patterns in long-chain ketone compositions for palaeotemperature assessment [J]. Nature, 1987, 330(6146): 367-369. doi: 10.1038/330367a0
[7] Boon J J, Leeuw J W, Burlingame A L B. Organic geochemistry of Walvis Bay diatomaceous ooze-III. Structural analysis of the monoenoic and polycyclic fatty acids [J]. Geochimica et Cosmochimica Acta, 1978, 42(6): 631-644. doi: 10.1016/0016-7037(78)90008-X
[8] 邢磊, 杨欣欣, 肖睿. 长链烯酮的组合特征及其对盐度和母源种属指示意义的研究进展[J]. 中国海洋大学学报(自然科学版), 2019, 49(10):79-87 doi: 10.16441/j.cnki.hdxb.20190247
XING Lei, YANG Xinxin, XIAO Rui. Progress of compositions and indications of long-chain alkenones [J]. Periodical of Ocean University of China, 2019, 49(10): 79-87. doi: 10.16441/j.cnki.hdxb.20190247
[9] 马晓旭, 刘传联, 金晓波, 等. 长链烯酮在古大气二氧化碳分压重建的应用[J]. 地球科学进展, 2019, 34(3):265-274 doi: 10.11867/j.issn.1001-8166.2019.03.0265
MA Xiaoxu, LIU Chuanlian, JIN Xiaobo, et al. The application of alkenone-based pCO2 reconstructions [J]. Advances in Earth Science, 2019, 34(3): 265-274. doi: 10.11867/j.issn.1001-8166.2019.03.0265
[10] Theroux S, D'Andrea W J, Toney J, et al. Phylogenetic diversity and evolutionary relatedness of alkenone-producing haptophyte algae in lakes: Implications for continental paleotemperature reconstructions[J]. Earth and Planetary Science Letters, 2010, 300(3–4): 311–320.
[11] Salacup J M, Farmer J R, Herbert T D, et al. Alkenone Paleothermometry in Coastal Settings: Evaluating the Potential for Highly Resolved Time Series of Sea Surface Temperature [J]. Paleoceanography and Paleoclimatology, 2019, 34(2): 164-181. doi: 10.1029/2018PA003416
[12] Longo W M, Huang Y, Yao Y, et al. Widespread occurrence of distinct alkenones from Group I haptophytes in freshwater lakes: Implications for paleotemperature and paleoenvironmental reconstructions [J]. Earth and Planetary Science Letters, 2018, 492: 239-250. doi: 10.1016/j.jpgl.2018.04.002
[13] Plancq J, Couto J M, Ijaz U Z, et al. Next-Generation Sequencing to Identify Lacustrine Haptophytes in the Canadian Prairies: Significance for Temperature Proxy Applications [J]. Journal of Geophysical Research:Biogeosciences, 2019, 124(7): 2144-2158. doi: 10.1029/2018JG004954
[14] Castañeda I S, Schouten S. A review of molecular organic proxies for examining modern and ancient lacustrine environments[J]. Quaternary Science Reviews, Elsevier Ltd, 2011, 30(21–22): 2851–2891.
[15] Brassell S C, Eglinton G, Marlowe I T, et al. Molecular stratigraphy: A new tool for climatic assessment [J]. Nature, 1986, 320(6058): 129-133. doi: 10.1038/320129a0
[16] Prahl F G, Muehlhausen L A, Zahnle D L. Further evaluation of long-chain alkenones as indicators of paleoceanographic conditions [J]. Geochimica et Cosmochimica Acta, 1988, 52(9): 2303-2310. doi: 10.1016/0016-7037(88)90132-9
[17] Müller P J, Kirst G, Ruhland G, et al. Calibration of the alkenone paleotemperature index
$ {\rm U}^{\rm K}_{37} $ [18] Bendle J, Rosell-Melé A. Distributions of
${\rm U}^{\rm K}_{37} $ ${\rm U}^{\rm {K'}}_{37} $ [19] Sikes E L, Farrington J W, Keigwin L D. Use of the alkenone unsaturation ratio
$ {\rm U}^{\rm K}_{37} $ [20] Jonas A S, Schwark L, Bauersachs T. Late Quaternary water temperature variations of the Northwest Pacific based on the lipid paleothermometers
${\rm{TEX}}^{\rm H}_{86} $ $ {\rm U}^{\rm {K'}}_{37} $ [21] Max L, Lembke-Jene L, Zou J, et al. Evaluation of reconstructed sea surface temperatures based on
$ {\rm U}^{\rm K}_{37} $ [22] Epstein B L, D'Hondt S, Quinn J G, et al. An effect of dissolved nutrient concentrations on alkenonebased temperature estimates [J]. Paleoceanography, 1998, 13(2): 122-126. doi: 10.1029/97PA03358
[23] Versteegh G J M, Riegman R, De Leeuw J W, et al.
$ {\rm U}^{\rm {K'}}_{37} $ [24] Hoefs M J L, Versteegh G J M, Rijpstra W F C, et al. Postdepositional oxic degradation of alkenones: Implications for the measurement of palaeo sea surface temperatures [J]. Paleoceanography, 1998, 13(1): 42-49. doi: 10.1029/97PA02893
[25] Rosell-Melé A, Comes P, Müller P J, et al. Alkenone fluxes and anomalous
${\rm U}^{\rm {K'}}_{37} $ [26] Harada N, Sato M, Shiraishi A, et al. Characteristics of alkenone distributions in suspended and sinking particles in the northwestern North Pacific [J]. Geochimica et Cosmochimica Acta, 2006, 70(8): 2045-2062. doi: 10.1016/j.gca.2006.01.024
[27] Seki O, Nakatsuka T, Kawamura K, et al. Time-series sediment trap record of alkenones from the western Sea of Okhotsk[J]. Marine Chemistry, 2007, 104(3–4): 253–265.
[28] Conte M H, Sicre M A, Rühlemann C, et al. Global temperature calibration of the alkenone unsaturation index (
${\rm U}^{\rm {K}}_{37} $ [29] Bijma J, Altabet M, Conte M, et al. Primary signal: Ecological and environmental factors-Report from Working Group 2 [J]. Geochemistry, Geophysics, Geosystems, 2001, 2(1): 2000GC000051.
[30] Sires E L, Volkman J K. Calibration of alkenone unsaturation ratios (
${\rm U}^{\rm {K'}}_{37} $ [31] Pelejero C, Calvo E. The upper end of the
${\rm U}^{\rm {K'}}_{37} $ [32] Bard E. Comparison of alkenone estimates with other paleotemperature proxies [J]. Geochemistry, Geophysics, Geosystems, 2001, 2(1): 2000GC000050.
[33] Hopmans E C, Schouten S, Pancost R D, et al. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2000, 14(7).
[34] 葛黄敏, 张传伦. 中国边缘海环境中GDGT的研究进展[J]. 中国科学: 地球科学, 2016, 46(4): 473–488
GE Huanmin, ZHANG Chuanlun. Advances in GDGT research in Chinese Marginal Seas: A review. Science China Earth Sciences, 2016, 46(4): 473–488.
[35] 陈立雷, 李凤, 刘健. 海洋沉积物中GDGTs和长链二醇的古气候—环境指示意义研究进展[J]. 地球科学进展, 2019, 34(8):855-867
CHEN Lilei, LI Feng, LIU Jian. Advances in glycerol dialkyl glycerol tetraethers and long-chain alkyl diols in the marine sediments: Implications for paleoclimatic and paleoenvironmental changes [J]. Advances in Earth Science, 2019, 34(8): 855-867.
[36] Wuchter C, Schouten S, Coolen M J L, et al. Temperature-dependent variation in the distribution of tetraether membrane lipids of marine Crenarchaeota: Implications for TEX86 paleothermometry [J]. Paleoceanography, 2004, 19(4): 1-10.
[37] Greenwood P F, Brocks J J, Grice K, et al. Organic geochemistry and mineralogy. I. Characterisation of organic matter associated with metal deposits [J]. Ore Geology Reviews, 2013, 50: 1-27. doi: 10.1016/j.oregeorev.2012.10.004
[38] Blaga C I, Reichart G J, Heiri O, et al. Tetraether membrane lipid distributions in water-column particulate matter and sediments: A study of 47 European lakes along a north-south transect [J]. Journal of Paleolimnology, 2009, 41(3): 523-540. doi: 10.1007/s10933-008-9242-2
[39] Schouten S, Hopmans E C, Sinninghe Damsté J S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review [J]. Organic Geochemistry, Elsevier Ltd, 2013, 54: 19-61. doi: 10.1016/j.orggeochem.2012.09.006
[40] Pitcher A, Hopmans E C, Mosier A C, et al. Core and intact polar glycerol dibiphytanyl glycerol tetraether lipids of ammonia-oxidizing Archaea enriched from marine and estuarine sediments [J]. Applied and Environmental Microbiology, 2011, 77(10): 3468-3477. doi: 10.1128/AEM.02758-10
[41] Sinninghe Damsté J S, Ossebaar J, Schouten S, et al. Distribution of tetraether lipids in the 25-ka sedimentary record of Lake Challa: Extracting reliable TEX86 and MBT/CBT palaeotemperatures from an equatorial African lake [J]. Quaternary Science Reviews, 2012, 50: 43-54. doi: 10.1016/j.quascirev.2012.07.001
[42] 赵美训, 李大伟, 邢磊. 古菌生物标志物古海水温度指标TEX86研究进展[J]. 海洋地质与第四纪地质, 2009, 29(3):75-84
ZHAO Meixun, LI Dawei, XING Lei. Using archaea biomarker index TEX86 as a paleo-sea surface temperature proxy [J]. Marine Geology & Quaternary Geology, 2009, 29(3): 75-84.
[43] Schouten S, Hopmans E C, Schefuß E, et al. Distributional variations in marine crenarchaeotal membrane lipids: A new tool for reconstructing ancient sea water temperatures?[J]. Earth and Planetary Science Letters, 2002, 204(1–2): 265–274.
[44] Sluijs A, Schouten S, Pagani M, et al. Subtropical Arctic Ocean temperatures during the Palaeocene/Eocene thermal maximum [J]. Nature, 2006, 441(7093): 610-613. doi: 10.1038/nature04668
[45] Kim J H, Schouten S, Hopmans E C, et al. Global sediment core-top calibration of the TEX86 paleothermometer in the ocean [J]. Geochimica et Cosmochimica Acta, 2008, 72(4): 1154-1173. doi: 10.1016/j.gca.2007.12.010
[46] Trommer G, Siccha M, van der Meer M T J, et al. Distribution of Crenarchaeota tetraether membrane lipids in surface sediments from the Red Sea [J]. Organic Geochemistry, Elsevier Ltd, 2009, 40(6): 724-731. doi: 10.1016/j.orggeochem.2009.03.001
[47] Liu Z, Pagani M, Zinniker D, et al. Global cooling during the eocene-oligocene climate transition[J]. 2009, 323(5918): 1187–1190.
[48] Kim J H, van der Meer J, Schouten S, et al. New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: Implications for past sea surface temperature reconstructions [J]. Geochimica et Cosmochimica Acta, 2010, 74(16): 4639-4654. doi: 10.1016/j.gca.2010.05.027
[49] Hopmans E C, Weijers J W H, Schefuß E, et al. A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids[J]. Earth and Planetary Science Letters, 2004, 224(1–2): 107–116.
[50] 于晓果, 边叶萍, 阮小燕, 等. 北冰洋沉积物中四醚脂类来源与TEX86指数初步研究[J]. 海洋地质与第四纪地质, 2015, 35(3):11-22
YU Xiaoguo, BIAN Yeping, RUAN Xiaoyan, et al. Glycerol dialkyl glyceroltetraethers and TEX86 index in surface sediments of the arctic ocean and the bering sea [J]. Marine Geology & Quaternary Geology, 2015, 35(3): 11-22.
[51] Xu Y, Jia Z, Xiao W, et al. Glycerol dialkyl glycerol tetraethers in surface sediments from three Pacific trenches: Distribution, source and environmental implications [J]. Organic Geochemistry, Elsevier Ltd, 2020, 147: 104079. doi: 10.1016/j.orggeochem.2020.104079
[52] Jenkyns H C, Schouten-Huibers L, Schouten S, et al. Warm Middle Jurassic-Early Cretaceous high-latitude sea-surface temperatures from the Southern Ocean [J]. Climate of the Past, 2012, 8(1): 215-225. doi: 10.5194/cp-8-215-2012
[53] Wade B S, Houben A J P, Quaijtaal W, et al. Multiproxy record of abrupt sea-surface cooling across the Eocene-Oligocene transition in the Gulf of Mexico [J]. Geology, 2012, 40(2): 159-162. doi: 10.1130/G32577.1
[54] Schouten S, Hopmans E C, Forster A, et al. Extremely high sea-surface temperatures at low latitudes during the middle Cretaceous as revealed by archaeal membrane lipids [J]. Geology, 2003, 31(12): 1069-1072. doi: 10.1130/G19876.1
[55] Tierney J E, Russell J M, Huang Y, et al. Northern hemisphere controls on tropical southeast African climate during the past 60 000 years [J]. Science, 2008, 322(5899): 252-255. doi: 10.1126/science.1160485
[56] Herfort L, Schouten S, Boon J P, et al. Application of the TEX86 temperature proxy to the southern North Sea [J]. Organic Geochemistry, 2006, 37(12): 1715-1726. doi: 10.1016/j.orggeochem.2006.07.021
[57] Menzel D, Hopmans E C, Schouten S, et al. Membrane tetraether lipids of planktonic Crenarchaeota in Pliocene sapropels of the eastern Mediterranean Sea[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2006, 239(1–2): 1–15.
[58] Jia G, Zhang J, Chen J, et al. Archaeal tetraether lipids record subsurface water temperature in the South China Sea [J]. Organic Geochemistry, 2012, 50: 68-77. doi: 10.1016/j.orggeochem.2012.07.002
[59] Pitcher A, Rychlik N, Hopmans E C, et al. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic Group I. 1b Archaeon [J]. ISME Journal, 2010, 4(4): 542-552. doi: 10.1038/ismej.2009.138
[60] Sinninghe Damsté J S. Spatial heterogeneity of sources of branched tetraethers in shelf systems: The geochemistry of tetraethers in the Berau River delta (Kalimantan, Indonesia) [J]. Geochimica et Cosmochimica Acta, 2016, 186: 13-31. doi: 10.1016/j.gca.2016.04.033
[61] Blumenberg M, Seifert R, Reitner J, et al. Membrane lipid patterns typify distinct anaerobic methanotrophic consortia [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(30): 11111-11116. doi: 10.1073/pnas.0401188101
[62] Schouten S, Hopmans E C, Sinninghe Damsté J S. The effect of maturity and depositional redox conditions on archaeal tetraether lipid palaeothermometry [J]. Organic Geochemistry, 2004, 35(5): 567-571. doi: 10.1016/j.orggeochem.2004.01.012
[63] Sinninghe Damsté J S, Strous M, Rijpstra W I C, et al. Linearly concatenated cyclobutane lipids form a dense bacterial membrane [J]. Nature, 2002, 419(6908): 708-712. doi: 10.1038/nature01128
[64] 曹亚俐, 赵宗山, 赵美训. 梯烷脂在海洋厌氧氨氧化研究中的应用进展[J]. 海洋地质与第四纪地质, 2013, 33(3):159-169
CAO Yali, ZHAO Zongshan, ZHAO Meixun. The application process og ladderane lipidsas biomarkers on the study of marine anaerobic ammonium oxidation [J]. Marine Geology & Quaternary Geology, 2013, 33(3): 159-169.
[65] Rattray J E. Ladderane Lipids in Anammox Bacteria: Occurence, Biosynthesis and Application as Environmental Markers[M]. Geologica Ultraiectina, 2008, 288(288).
[66] Kartal B, De Almeida N M, Maalcke W J, et al. How to make a living from anaerobic ammonium oxidation [J]. FEMS Microbiology Reviews, 2013, 37(3): 428-461. doi: 10.1111/1574-6976.12014
[67] Chaban V V. , Nielsen M B, Kopec W, et al. Insights into the role of cyclic ladderane lipids in bacteria from computer simulations [J]. Chemistry and Physics of Lipids, 2014, 181: 76-82. doi: 10.1016/j.chemphyslip.2014.04.002
[68] Wakeham S G, Turich C, Schubotz F, et al. Biomarkers, chemistry and microbiology show chemoautotrophy in a multilayer chemocline in the Cariaco Basin [J]. Deep-Sea Research Part I:Oceanographic Research Papers, 2012, 63: 133-156. doi: 10.1016/j.dsr.2012.01.005
[69] Han P, Gu J D. More refined diversity of anammox bacteria recovered and distribution in different ecosystems [J]. Applied Microbiology and Biotechnology, 2013, 97(8): 3653-3663. doi: 10.1007/s00253-013-4756-6
[70] Rattray J E, Van De Vossenberg J, Hopmans E C, et al. Ladderane lipid distribution in four genera of anammox bacteria [J]. Archives of Microbiology, 2008, 190(1): 51-66. doi: 10.1007/s00203-008-0364-8
[71] Rattray J E, Van Vossenberg J De, Jaeschke A, et al. Impact of temperature on ladderane lipid distribution in anammox bacteria [J]. Applied and Environmental Microbiology, 2010, 76(5): 1596-1603. doi: 10.1128/AEM.01796-09
[72] Zhao Z, Cao Y, Li L, et al. Sedimentary ladderane core lipids as potential indicators of hypoxia in the East China Sea [J]. Chinese Journal of Oceanology and Limnology, 2013, 31(1): 237-244. doi: 10.1007/s00343-013-1308-y
[73] Jaescnke A, Abbas B, Zabel M, et al. Molecular evidence for anaerobic ammonium-oxidizing (anammox) bacteria in continental shelf and slope sediments off northwest Africa [J]. Limnology and Oceanography, 2010, 55(1): 365-376. doi: 10.4319/lo.2010.55.1.0365
[74] Kuypers M M M, Silekers A O, Lavik G, et al. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea [J]. Nature, 2003, 422(6932): 608-611. doi: 10.1038/nature01472
[75] Jaeschke A, Rooks C, Trimmer M, et al. Comparison of ladderane phospholipid and core lipids as indicators for anaerobic ammonium oxidation (anammox) in marine sediments [J]. Geochimica et Cosmochimica Acta, 2009, 73(7): 2077-2088. doi: 10.1016/j.gca.2009.01.013
[76] 曹亚俐. 利用生物标志物梯烷脂研究东海厌氧氨氧化活动的时空分布特征[D]. 中国海洋大学, 2013
CAO Yali. The study on the spatial and temporal distributions of anammox in the East China Sea by ladderane lipids[D]. Ocean University of China, 2013
[77] 侯笛, 张俊杰, 邢磊, 等. 长链烷基二醇在海洋环境重建中的研究进展[J]. 地球科学进展, 2019, 34(2):140-147 doi: 10.11867/j.issn.1001-8166.2019.02.0140
HOU Di, ZHANG Junjie, XING Lei, et al. Progress of long-chain alkyl diols in marine environmental reconstruction [J]. Advances in Earth Science, 2019, 34(2): 140-147. doi: 10.11867/j.issn.1001-8166.2019.02.0140
[78] De Bar M W, Hopmans E C, Verweij M, et al. Development and comparison of chromatographic methods for the analysis of long chain diols and alkenones in biological materials and sediment [J]. Journal of Chromatography A, 2017, 1521: 150-160. doi: 10.1016/j.chroma.2017.09.037
[79] De Leeuw J W, Irene W, Rijpstra C, et al. The occurrence and identification of C30, C31 and C32 alkan-1, 15-diols and alkan-15-one-1-ols in Unit I and Unit II Black Sea sediments [J]. Geochimica et Cosmochimica Acta, 1981, 45(11): 2281-2285. doi: 10.1016/0016-7037(81)90077-6
[80] Rampen S W, Willmott V, Kim J H, et al. Long chain 1, 13- and 1, 15-diols as a potential proxy for palaeotemperature reconstruction [J]. Geochimica et Cosmochimica Acta, 2012, 84: 204-216. doi: 10.1016/j.gca.2012.01.024
[81] Volkman J K, Barrett S M, Dunstan G A, et al. C30-C32 alkyl diols and unsaturated alcohols in microalgae of the class Eustigmatophyceae [J]. Organic Geochemistry, 1992, 18(1): 131-138. doi: 10.1016/0146-6380(92)90150-V
[82] Volkman J K, Barrett S M, Blackburn S I. Eustigmatophyte microalgae are potential sources of C29 sterols, C22-C28 n-alcohols and C28-C32 n-alkyl diols in freshwater environments [J]. Organic Geochemistry, 1999, 30(5): 307-318. doi: 10.1016/S0146-6380(99)00009-1
[83] Rampen S W, Willmott V, Kim J H, et al. Evaluation of long chain 1, 14-alkyl diols in marine sediments as indicators for upwelling and temperature [J]. Organic Geochemistry, 2014, 76: 39-47. doi: 10.1016/j.orggeochem.2014.07.012
[84] Fawley K P, Fawley M W. Observations on the Diversity and Ecology of Freshwater Nannochloropsis (Eustigmatophyceae), with Descriptions of New Taxa [J]. Protist, 2007, 158(3): 325-336. doi: 10.1016/j.protis.2007.03.003
[85] Sinninghe Damsté J S, Rampen S, Irene W, et al. A diatomaceous origin for long-chain diols and mid-chain hydroxy methyl alkanoates widely occurring in quaternary marine sediments: Indicators for high-nutrient conditions [J]. Geochimica et Cosmochimica Acta, 2003, 67(7): 1339-1348. doi: 10.1016/S0016-7037(02)01225-5
[86] Rampen S W, Schouten S, Sinninghe Damsté J S. Occurrence of long chain 1, 14-diols in Apedinella radians [J]. Organic Geochemistry, 2011, 42(5): 572-574. doi: 10.1016/j.orggeochem.2011.03.009
[87] De Bar M W, Ullgren J E, Thunnell R C, et al. Long-chain diols in settling particles in tropical oceans: Insights into sources, seasonality and proxies [J]. Biogeosciences, 2019, 16(8): 1705-1727. doi: 10.5194/bg-16-1705-2019
[88] Yang Y, Ruan X, Gao C, et al. Assessing the applicability of the long-chain diol (LDI) temperature proxy in the high-temperature South China Sea [J]. Organic Geochemistry, Elsevier Ltd, 2020, 144: 104017. doi: 10.1016/j.orggeochem.2020.104017
[89] De Bar M W, Weiss G, Yildiz C, et al. Global temperature calibration of the Long chain Diol Index in marine surface sediments [J]. Organic Geochemistry, Elsevier Ltd, 2020, 142: 103983. doi: 10.1016/j.orggeochem.2020.103983
[90] Naafs B D A, Hefter J, Stein R. Application of the long chain diol index (LDI) paleothermometer to the early Pleistocene (MIS 96) [J]. Organic Geochemistry, 2012, 49: 83-85. doi: 10.1016/j.orggeochem.2012.05.011
[91] Zhu X, Jia G, Mao S, et al. Sediment records of long chain alkyl diols in an upwelling area of the coastal northern South China Sea [J]. Organic Geochemistry, 2018, 121: 1-9. doi: 10.1016/j.orggeochem.2018.03.014
[92] Zhu X, Mao S, Sun Y, et al. Long chain diol index (LDI) as a potential measure to estimate annual mean sea surface temperature in the northern South China Sea [J]. Estuarine, Coastal and Shelf Science, 2019, 221: 1-7. doi: 10.1016/j.ecss.2019.03.012
[93] De Bar M W, Dorhout D J C, Hopmans E C, et al. Constraints on the application of long chain diol proxies in the Iberian Atlantic margin [J]. Organic Geochemistry, 2016, 101: 184-195. doi: 10.1016/j.orggeochem.2016.09.005
[94] Rodrigo-Gámiz M, Rampen S W, Schouten S, et al. The impact of oxic degradation on long chain alkyl diol distributions in Arabian Sea surface sediments [J]. Organic Geochemistry, 2016, 100: 1-9. doi: 10.1016/j.orggeochem.2016.07.003
[95] Rodrigo-Gámiz M, Rampen S W, De Haas H, et al. Constraints on the applicability of the organic temperature proxies
${\rm U}^{\rm {K'}}_{37} $ [96] He L, Kang M, Zhang D, et al. Evaluation of environmental proxies based on long chain alkyl diols in the East China Sea [J]. Organic Geochemistry, 2020, 139: 103948. doi: 10.1016/j.orggeochem.2019.103948
[97] Lipp J S, Hinrichs K U. Structural diversity and fate of intact polar lipids in marine sediments [J]. Geochimica et Cosmochimica Acta, 2009, 73(22): 6816-6833. doi: 10.1016/j.gca.2009.08.003
[98] Liu X L, Summons R E, Hinrichs K U. Extending the known range of glycerol ether lipids in the environment: Structural assignments based on tandem mass spectral fragmentation patterns [J]. Rapid Communications in Mass Spectrometry, 2012, 26(19): 2295-2302. doi: 10.1002/rcm.6355
[99] Fietz S, Huguet C, Rueda G, et al. Hydroxylated isoprenoidal GDGTs in the Nordic Seas [J]. Marine Chemistry, 2013, 152: 1-10. doi: 10.1016/j.marchem.2013.02.007
[100] Kaiser J, Arz H W. Sources of sedimentary biomarkers and proxies with potential paleoenvironmental significance for the Baltic Sea [J]. Continental Shelf Research, 2016, 122: 102-119. doi: 10.1016/j.csr.2016.03.020
[101] Elling F J, Könneke M, Mußmann M, et al. Influence of temperature, pH, and salinity on membrane lipid composition and TEX86 of marine planktonic thaumarchaeal isolates [J]. Geochimica et Cosmochimica Acta, 2015, 171: 238-255. doi: 10.1016/j.gca.2015.09.004
[102] Elling F J, Könneke M, Lipp J S, et al. Effects of growth phase on the membrane lipid composition of the thaumarchaeon Nitrosopumilus maritimus and their implications for archaeal lipid distributions in the marine environment [J]. Geochimica et Cosmochimica Acta, 2014, 141: 579-597. doi: 10.1016/j.gca.2014.07.005
[103] Liu X L, Lipp J S, Simpson J H, et al. Mono- and dihydroxyl glycerol dibiphytanyl glycerol tetraethers in marine sediments: Identification of both core and intact polar lipid forms [J]. Geochimica et Cosmochimica Acta, 2012, 89: 102-115. doi: 10.1016/j.gca.2012.04.053
[104] Lin Y S, Lipp J S, Elvert M, et al. Assessing production of the ubiquitous archaeal diglycosyl tetraether lipids in marine subsurface sediment using intramolecular stable isotope probing [J]. Environmental Microbiology, 2013, 15(5): 1634-1646. doi: 10.1111/j.1462-2920.2012.02888.x
[105] Sinninghe Damsté J S, Rijpstra W I C, Hopmans E C, et al. Intact polar and core glycerol dibiphytanyl glycerol tetraether lipids of group I. 1a and I. 1b Thaumarchaeota in soil [J]. Applied and Environmental Microbiology, 2012, 78(19): 6866-6874. doi: 10.1128/AEM.01681-12
[106] Huguet C, Fietz S, Rosell-Melé A. Global distribution patterns of hydroxy glycerol dialkyl glycerol tetraethers [J]. Organic Geochemistry, 2013, 57: 107-118. doi: 10.1016/j.orggeochem.2013.01.010
[107] Lü X, Liu X L, Elling F J, et al. Hydroxylated isoprenoid GDGTs in Chinese coastal seas and their potential as a paleotemperature proxy for mid-to-low latitude marginal seas[J]. Organic Geochemistry, Elsevier Ltd, 2015, 89–90: 31–43.
[108] Fietz S, Ho S L, Huguet C, et al. Appraising GDGT-based seawater temperature indices in the Southern Ocean [J]. Organic Geochemistry, 2016, 102: 93-105. doi: 10.1016/j.orggeochem.2016.10.003
[109] Park E, Hefter J, Fischer G, et al. Seasonality of archaeal lipid flux and GDGT-based thermometry in sinking particles of high-latitude oceans: Fram Strait (79°N) and Antarctic Polar Front (50°S) [J]. Biogeosciences, 2019, 16(11): 2247-2268. doi: 10.5194/bg-16-2247-2019
[110] Davtian N, Ménot G, Fagault Y, et al. Western Mediterranean Sea Paleothermometry Over the Last Glacial Cycle Based on the Novel RI-OH Index [J]. Paleoceanography and Paleoclimatology, 2019, 34(4): 616-634. doi: 10.1029/2018PA003452
[111] Kang S, Shin K H, Kim J H. Occurrence and distribution of hydroxylated isoprenoid glycerol dialkyl glycerol tetraethers (OH-GDGTs) in the Han River system, South Korea [J]. Acta Geochimica, 2017, 36(3): 367-369. doi: 10.1007/s11631-017-0165-3
[112] Wei B, Jia G, Hefter J, et al. Comparison of the
${\rm U}^{\rm {K'}}_{37} $ ${\rm {TEX}}^{\rm {H}}_{86} $ [113] Yang Y, Gao C, Dang X, et al. Assessing hydroxylated isoprenoid GDGTs as a paleothermometer for the tropical South China Sea [J]. Organic Geochemistry, 2018, 115: 156-165. doi: 10.1016/j.orggeochem.2017.10.014
[114] Wang C, Bendle J, Yang Y, et al. Impacts of pH and temperature on soil bacterial 3-hydroxy fatty acids: Development of novel terrestrial proxies [J]. Organic Geochemistry, 2016, 94: 21-31. doi: 10.1016/j.orggeochem.2016.01.010
[115] 孙棋棋, 宋金明, 袁华茂, 等. 细菌源3-羟基脂肪酸作为环境变化代用指标的研究进展[J]. 海洋科学, 2021, 45(8):98-108
SUN Qiqi, SONG Jinming, YUAN Huanmao, et al. Bacterial 3-hydroxy fatty acids as a biomarker of environmental change [J]. Marin Sciences, 2021, 45(8): 98-108.
[116] Kumar G S, Jagannadham M V. , Ray M K. Low-temperature-induced changes in composition and fluidity of lipopolysaccharides in the antarctic psychrotrophic bacterium Pseudomonas syringae [J]. Journal of Bacteriology, 2002, 184(23): 6746-6749. doi: 10.1128/JB.184.23.6746-6749.2002
[117] Raetz C R H, Reynolds C M, Trent M S, et al. Lipid a modification systems in gram-negative bacteria [J]. Annual Review of Biochemistry, 2007, 76: 295-329. doi: 10.1146/annurev.biochem.76.010307.145803
[118] Cranwell P A. Diagenesis of free and bound lipids in terrestrial detritus deposited in a lacustrine sediment [J]. Organic Geochemistry, 1981, 3(3): 79-89. doi: 10.1016/0146-6380(81)90002-4
[119] Yang Y, Wang C, Bendle J A, et al. Appraisal of paleoclimate indices based on bacterial 3-hydroxy fatty acids in 20 Chinese alkaline lakes [J]. Organic Geochemistry, 2021, 160: 104277. doi: 10.1016/j.orggeochem.2021.104277
[120] Yang Y, Wang C, Bendle J A, et al. A new sea surface temperature proxy based on bacterial 3-hydroxy fatty acids [J]. Organic Geochemistry, 2020, 141: 104277.
[121] Sjögren J, Magnusson J, Broberg A, et al. Antifungal 3-Hydroxy Fatty Acids from Lactobacillus plantarum MiLAB 14 [J]. Applied and Environmental Microbiology, 2003, 69(12): 7554-7557. doi: 10.1128/AEM.69.12.7554-7557.2003
[122] Wakeham S G. Monocarboxylic, dicarboxylic and hydroxy acids released by sequential treatments of suspended particles and sediments of the Black Sea [J]. Organic Geochemistry, 1999, 30(9): 1059-1074. doi: 10.1016/S0146-6380(99)00084-4
[123] Matsumoto G I, Nagashima H. Occurrence of 3-hydroxy acids in microalgae and cyanobacteria and their geochemical significance [J]. Geochimica et Cosmochimica Acta, 1984, 48(8): 1683-1687. doi: 10.1016/0016-7037(84)90337-5
[124] Huguet A, Coffinet S, Roussel A, et al. Evaluation of 3-hydroxy fatty acids as a pH and temperature proxy in soils from temperate and tropical altitudinal gradients [J]. Organic Geochemistry, 2019, 129: 1-13. doi: 10.1016/j.orggeochem.2019.01.002
[125] Véquaud P, Derenne S, Thibault A, et al. Development of global temperature and pH calibrations based on bacterial 3-hydroxy fatty acids in soils [J]. Biogeosciences, 2021, 18(12): 3937-3959. doi: 10.5194/bg-18-3937-2021
[126] Mauel M J, Giovannoni S J, Fryer J L. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing [J]. Diseases of Aquatic Organisms, 1999, 35(2): 115-123.
[127] Wakeham S G, Pease T K, Benner R. Hydroxy fatty acids in marine dissolved organic matter as indicators of bacterial membrane material [J]. Organic Geochemistry, 2003, 34(6): 857-868. doi: 10.1016/S0146-6380(02)00189-4
[128] Wang C, Bendle J A, Zhang H, et al. Holocene temperature and hydrological changes reconstructed by bacterial 3-hydroxy fatty acids in a stalagmite from central China [J]. Quaternary Science Reviews, 2018, 192: 97-105. doi: 10.1016/j.quascirev.2018.05.030
[129] Peterse F, van der Meer J, Schouten S, et al. Revised calibration of the MBT-CBT paleotemperature proxy based on branched tetraether membrane lipids in surface soils [J]. Geochimica et Cosmochimica Acta, 2012, 96: 215-229. doi: 10.1016/j.gca.2012.08.011
[130] Bartlett M G, Chapman D S, Harris R N. A decade of ground-air temperature tracking at emigrant pass observatory, Utah [J]. Journal of Climate, 2006, 19(15): 3722-3731. doi: 10.1175/JCLI3808.1
[131] Siles J A, Margesin R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? [J]. Microbial Ecology, 2016, 72(1): 207-220. doi: 10.1007/s00248-016-0748-2
[132] Margesin R, Jud M, Tscherko D, et al. Microbial communities and activities in alpine and subalpine soils [J]. FEMS Microbiology Ecology, 2009, 67(2): 208-218. doi: 10.1111/j.1574-6941.2008.00620.x
-




 下载:
下载:


 指标
指标