Aluminum Concentrate Prepared by Activated Roasting-Secondary Alkaline Leaching Desilication of Pyrite Tailings
-
摘要:
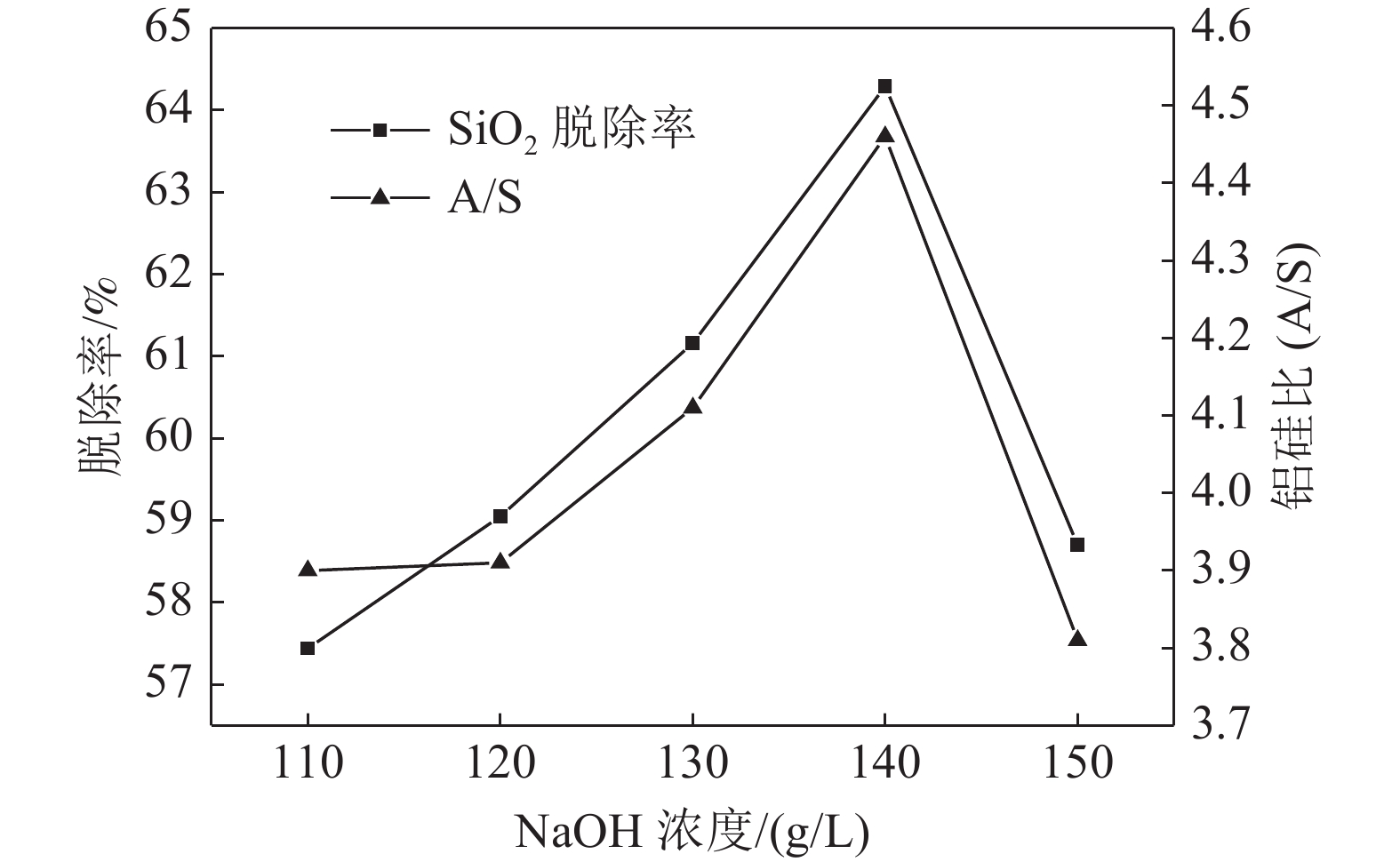
这是一篇冶金工程领域的论文。西南某地区硫铁矿选硫尾矿中的氧化铝含量高于46%,具有较高回收利用价值,但尾矿中的铝硅酸盐矿物中存在含量较高且晶体结构稳定的SiO2,导致尾矿的铝硅比(A/S)较低,仅为1.72。为了实现铝硅分离,制得铝精矿以回收利用该尾矿,通过活化焙烧使其中结构稳定的SiO2转变为非晶态SiO2,再使用NaOH溶液二次浸出焙砂脱硅制备得到铝精矿。结果表明:在焙烧时间35 min,焙烧温度1140 ℃,一次碱浸NaOH浓度140 g/L,浸出温度110 ℃,浸出时间30 min,浸出液固比16的适宜条件下,铝硅比(A/S)提高到4.11;在二次碱浸NaOH浓度140 g/L、浸出温度110 ℃、浸出时间30 min、浸出液固比10的较佳条件下,得到铝硅比(A/S)为5.11的铝精矿,为该尾矿的综合利用打下了良好的基础。
Abstract:This is an essay in the field of metallurgical engineering. Content of alumina in pyrite tailings in the southwest China is higher than 46%, and the alumina is of high recycling value. However, SiO2 with high content and stable crystal structure exists in aluminosilicate minerals in tailings, resulting in a low aluminosilicate ratio of Al2O3/SiO2 (A/S) of tailings, which is only 1.72. To realize the separation of aluminum and silicon, and obtain aluminum concentrate for recycling of the tailings, the SiO2 with stable structure is transformed into amorphous SiO2 by activated roasting. And then the calcine is leached twice with NaOH solution for desilicating to obtain the aluminum concentrate. The results show that under the optimum conditions of roasting time of 35 min, roasting temperature of 1140 ℃, the concentration of NaOH 140 g/L in primary alkaline leaching, leaching temperature of 110 ℃, leaching time of 30 min, the ratio of liquid to solid 16, the aluminum-silicon ratio (A/S) increases to 4.11. Under the optimum conditions of NaOH concentration 140 g/L in secondary alkaline leaching, leaching temperature 105 ℃, leaching time 30 min, and leaching liquid to solid ratio 10, the aluminum-silicon ratio (A/S) of 5.11 is obtained, which lays a good foundation for comprehensive utilization of the tailings.
-
Key words:
- Metallurgical engineering /
- Pyrite tailings /
- Aluminum concentrate /
- Activation roasting /
- Desilication
-

-
表 1 硫铁矿尾矿化学多项分析结果/%
Table 1. Chemical multinomial analysis results of pyrite tailings
Al2O3 SiO2 TiO2 TFe S MgO CaO Sc2O3* TREO* Ga* Nb* 46.40 26.94 5.17 1.11 0.75 0.40 0.73 41.4 963 607 171 *单位为g/t。 表 2 硫铁矿尾矿的重要物相组成及含量/%
Table 2. Important phase composition and content of pyrite tailings
叶腊石 绿泥石 高岭石 一水硬铝石 三水铝石 锐钛矿 黄铁矿 海泡石 云母 石英 石墨 文石 石膏 板铁矿 41.9 0.6 11.2 20.8 4.5 4.6 1.5 0.6 7.9 1.7 0.4 0.4 - 1.1 表 3 焙砂一次碱浸脱硅产物成分/%
Table 3. Composition of desilication products from primary alkaline leaching of calcine
Al2O3 SiO2 TiO2 CaO Fe S TREO* Sc2O3* Ga* Nb* 63.61 15.47 7.40 1.08 1.66 0.10 1420 61 87.9 251 *单位为 g/t。 表 4 二次碱浸脱硅产物主要化学成分/%
Table 4. Main chemical composition of secondary alkaline leaching desilication products
Al2O3 SiO2 TiO2 CaO Fe S TREO* Sc2O3* Ga* Nb* 68.37 13.39 8.21 1.22 1.85 0.05 1572 66 96 279 *单位为 g/t。 表 5 二次碱浸脱硅溶液及洗液的主要化学成分/(g/L)
Table 5. Main chemical components of secondary alkaline leaching desilication solution and lotion
Al2O3 SiO2 NaOH Ga* Sc* Nb* TREO* 浸出液 1.30 3.40 141.6 0.12 0.01 <0.01 0.01 洗液 0.51 1.45 51.20 0.065 0.01 <0.01 0.01 *单位为 mg/L。 -
[1] 王庚亮. 硫铁矿在中国硫资源中的地位分析[J]. 化工矿产地质, 2018, 40(1):53-59. WANG G L. Analysis on pyrite’s status in sulfur resource of China[J]. Geology of Chemical Minerals, 2018, 40(1):53-59.
WANG G L. Analysis on pyrite’s status in sulfur resource of China[J]. Geology of Chemical Minerals, 2018, 40(1): 53-59.
[2] 李超, 刘述平, 唐湘萍. 某硫铁矿尾矿脱硅浸出液再生及硅酸钙的合成[J]. 有色金属(冶炼部分), 2019(2):25-28. LI C, LIU S P, TANG X P. Regeneration of desiliconization leachate from pyrite tailings and synthesis of calcium silicate[J]. Nonferrous Metals(Extractive Metallurgy), 2019(2):25-28.
LI C, LIU S P, TANG X P. Regeneration of desiliconization leachate from pyrite tailings and synthesis of calcium silicate[J]. Nonferrous Metals(Extractive Metallurgy), 2019(2): 25-28.
[3] 刘敬勇, 赵永久. 硫铁矿资源开采利用过程中的环境污染问题及控制对策[J]. 中国矿业, 2007, 16(7):55-57. LIU J Y, ZHAO Y J. Environmental pollution and countermeasures in pyrite resources exploration[J]. China Mining Magazine, 2007, 16(7):55-57.
LIU J Y, ZHAO Y J. Environmental pollution and countermeasures in pyrite resources exploration[J]. China Mining Magazine, 2007, 16(7): 55−57.
[4] 何兵兵, 刘代俊, 王章露, 等. 硫铁矿尾矿的综合利用工艺研究[J]. 硫磷设计与粉体工程, 2012(6):12-22. HE B B, LIU D J, WANG Z L, et al. Process study for comprehensive utilization of pyrite tailing[J]. Sulphur Phosphorus & Bulk Materials Handling Related Engineering, 2012(6):12-22. doi: 10.3969/j.issn.1009-1904.2012.06.010
HE B B, LIU D J, WANG Z L, et al. Process study for comprehensive utilization of pyrite tailing[J]. Sulphur Phosphorus & Bulk Materials Handling Related Engineering, 2012(6): 12-22. doi: 10.3969/j.issn.1009-1904.2012.06.010
[5] 李振宇. 西北某高硫铝土矿浮选脱硫实验研究[J]. 矿产综合利用, 2020(5):77-81. LI Z Y. Study on the flotation desulfurization of high-sulfur bauxite in Northwest China[J]. Multipurpose Utilization of Mineral Resources, 2020(5):77-81.
LI Z Y. Study on the flotation desulfurization of high-sulfur bauxite in Northwest China[J]. Multipurpose Utilization of Mineral Resources, 2020(5): 77-81.
[6] 刘佳囡, 曹诗圆, 程颖, 等. 铝土矿中铝、铁、硅高附加值综合利用研究[J]. 矿产综合利用, 2019(4):87-90. LIU J N, CAO S Y, CHENG Y, et al. Research on comprehensive utilization of aluminium, iron and silicon from bauxite[J]. Multipurpose Utilization of Mineral Resources, 2019(4):87-90.
LIU J N, CAO S Y, CHENG Y, et al. Research on comprehensive utilization of aluminium, iron and silicon from bauxite[J]. Multipurpose Utilization of Mineral Resources, 2019(4): 87-90.
[7] 王腾飞, 张金山, 李侠, 等. 碱法提取高铝粉煤灰中氧化铝的研究进展[J]. 矿产综合利用, 2019(1):16-21. WANG T F, ZHANG J S, LI X, et al. Research progress of extracting alumina in alkali method from high- alumina coal fly ash[J]. Multipurpose Utilization of Mineral Resources, 2019(1):16-21.
WANG T F, ZHANG J S, LI X, et al. Research progress of extracting alumina in alkali method from high- alumina coal fly ash[J]. Multipurpose Utilization of Mineral Resources, 2019(1): 16-21.
[8] 魏存弟, 赵峰, 马鸿文, 等. 叶蜡石加热相变及其演化特征[J]. 吉林大学学报(地球科学版), 2005, 35(2):150-154. WEI C D, ZHAO F, MA H W, et al. Heating phase transformation and evolutionary characteristics of pyrophyllite[J]. Journal of Jilin University:Earth Science Edition, 2005, 35(2):150-154.
WEI C D, ZHAO F, MA H W, et al. Heating phase transformation and evolutionary characteristics of pyrophyllite[J]. Journal of Jilin University: Earth Science Edition, 2005, 35(2): 150−154.
[9] 罗琳, 刘永康, 何伯泉. 一水硬铝石一高岭石型铝土矿焙烧脱硅热力学机理研究[J]. 有色金属, 1999, 51(1):25-29. LUO L, LIU Y K, HE B Q. Thermodynamic mechanism of roasting pre-desilication process of diasporic-kaolin type bauxite[J]. Nonferrous Metals, 1999, 51(1):25-29.
LUO L, LIU Y K, HE B Q. Thermodynamic mechanism of roasting pre-desilication process of diasporic-kaolin type bauxite[J]. Nonferrous Metals, 1999, 51(1): 25−29.
[10] 姜涛, 李光辉, 范晓慧, 等. 一水硬铝石型铝土矿焙烧碱浸脱硅新工艺(Ⅰ)[J]. 中国有色金属学报, 2000, 10(4):534-538. JIANG T, LI G H, FAN X H, et al. Desilication from diasporic bauxite by roasting-alkali leaching process (Ⅰ)[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(4):534-538. doi: 10.3321/j.issn:1004-0609.2000.04.016
JIANG T, LI G H, FAN X H, et al. Desilication from diasporic bauxite by roasting-alkali leaching process (Ⅰ)[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(4): 534−538. doi: 10.3321/j.issn:1004-0609.2000.04.016
[11] 李光辉, 姜 涛, 范晓慧, 等. 一水硬铝石型铝土矿焙烧碱浸脱硅新工艺(Ⅲ)[J]. 中国有色金属学报, 2000, 10(6):899-904. LI G H, JIANG T, FAN X H, et al. Technology of desilication from diasporic bauxite by roasting-alkali leaching process (Ⅲ)[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6):899-904. doi: 10.3321/j.issn:1004-0609.2000.06.028
LI G H, JIANG T, FAN X H, et al. Technology of desilication from diasporic bauxite by roasting-alkali leaching process (Ⅲ)[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(6): 899−904. doi: 10.3321/j.issn:1004-0609.2000.06.028
[12] 李光辉, 姜 涛, 范晓慧, 等. 伊利石中硅的热化学活化与脱除[J]. 金属矿山, 2004(7):18-22. LI G H, JIANG T, FAN X H, et al. Thermochemical actication of silicon in illite and its removal[J]. Metal Mine, 2004(7):18-22. doi: 10.3321/j.issn:1001-1250.2004.07.006
LI G H, JIANG T, FAN X H, et al. Thermochemical actication of silicon in illite and its removal[J]. Metal Mine, 2004(7): 18-22. doi: 10.3321/j.issn:1001-1250.2004.07.006
-




 下载:
下载: