Joint and Rapid Determination of 210Pb-210Bi-210Po in Rock, Soil and Sediment Samples by Constant Temperature Spontaneous Deposition on Cu-foil with Gross α and Gross β Counting
-
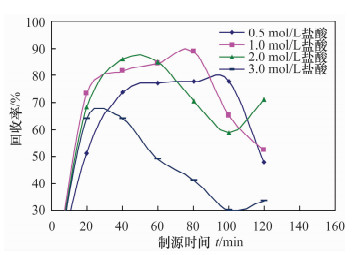
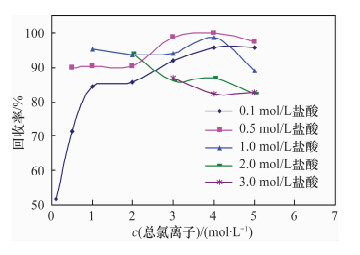
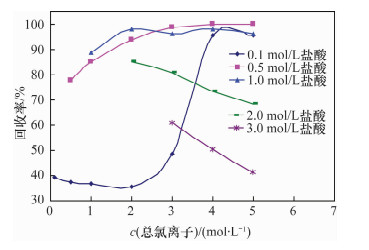
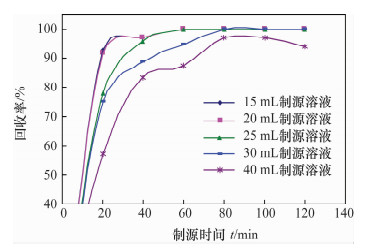
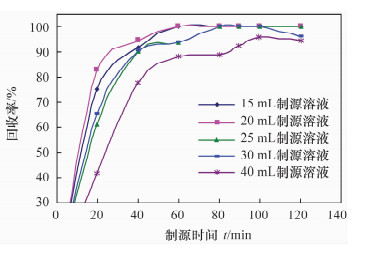
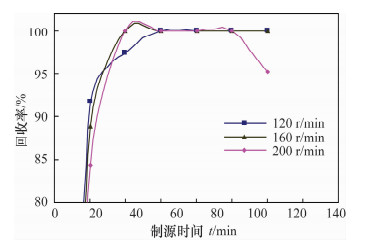
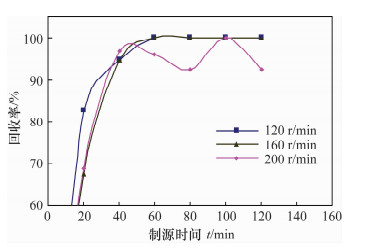
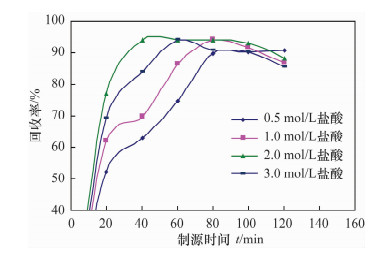
摘要: 铀系核素210Pb-210Bi-210Po目前通常采用相对独立的分析技术,三核素分别进行测定,并存在一些技术问题需要解决。文献报道的三核素联测技术需要使用多种昂贵的测试设备和示踪剂,或者制源体系抗干扰能力较弱且分析周期较长。本文研究了210Bi、210Po同时且定量恒温自沉积于铜箔的最佳制源条件,建立了双样-两次铜箔恒温自沉积制源-总α、总β同时计数法快速联合测定岩石、土壤及沉积物样品中210Pb-210Bi-210Po的分析技术。结果表明,当铜镀片面积为3.14 cm2,盐酸浓度为0.5 mol/L,氯化钠浓度为3.5 mol/L,溶液体积为20 mL,恒温90℃,振速为120 r/min,振幅20 mm,制源70 min,210Bi和210Po可同步定量自沉积且210Pb不沉积。在抗坏血酸存在下,大量共存元素不干扰自沉积。方法的精密度优于5%,全程加标放化回收率在99.5%~100.5%之间。该联测技术采用的制源体系抗稳定铋干扰能力较强,回收稳定,分析周期短,仅需一台国产测试设备并无需示踪剂即可完成三核素联合测定,同时也适合于三核素的独立测定。Abstract: Measurement techniques of uranium series nuclides 210Pb, 210Bi and 210Po are relatively independent at present for most cases, and there are still some technical issues that need to be overcome. Joint measurement techniques of these nuclides reported in the literature require expensive testing apparatus and radioactive tracers, or the capacity of resisting disturbance of the deposition conditions is relatively weak and the test period is relatively long. The study conducted of the optimum spontaneous deposition conditions of 210Bi and 210Po on Cu simultaneously and quantitatively is reported in this paper, and the establishment of a new joint measurement technique of 210Pb, 210Bi and 210Po in rock, soil and sediment samples. It was found that 210Bi and 210Po were deposited on Cu foil simultaneously, while 210Pb was not deposited on a 3.14 cm2 Cu foil under the conditions of 0.5 mol/L HCl, 3.5 mol/L NaCl in the total 20 mL solution at 90℃, with a vibration frequency of 120 r/min and vibration amplitude of 20 mm for 70 min. Due to the presence of ascorbic acid, lots of coexisting elements have no interference with target nuclides. The precision of this testing technology was higher than 5% and total recovery rates were 99.5%-100.5%. This new measurement technique has a strong capacity to resist Bi disturbance, the recovery rate is more constant than traditional methods and the test period is short with one set of home-made testing apparatus and no radioactive tracers required. Moreover, this measurement technique is also suitable for the determination of one of these three nuclides separately.
-
Key words:
- Cu /
- 210Pb /
- 210Bi /
- 210Po /
- double samples /
- joint measurement /
- gross α and β counting
-

-
表 1 共存元素(离子)允许量
Table 1. Permission amounts of coexisting elements or ions
共存元素 允许量/μg 共存元素(离子) 允许量/μg Au(Ⅲ) 2 Mn(Ⅶ) 2500 Te(Ⅳ) 5 Pb(Ⅱ) 20000 Ag(Ⅰ) 5 Fe(Ⅲ) 20000 Se(Ⅳ) 10 PO43- 150000 Hg(Ⅱ) 10 ClO4- 200000 Sb(Ⅲ) 10 F- 200000 Ce(Ⅳ) 10 NO3- 200000 Mo(Ⅵ) 100 SO42- 200000 V(Ⅴ) 200 238U(Ⅵ) 500 Bq Bi(Ⅲ) 200 232Th(Ⅳ) 500 Bq Cr(Ⅵ) 400 226Ra(Ⅱ) 500 Bq Cu(Ⅱ) 2000 40K(Ⅰ) 10000 Bq 表 2 全程加标放化回收率
Table 2. Total radiochemical spiked recovery rates
标准物质
编号样品名称 取样量/g 三核素
加标量
/Bq回收率/% 210Pb 210Bi 210Po GBW 07103 岩石 1.0000 0.10 99.6 100.4 100.2 GBW 07106 岩石 1.0000 0.10 99.6 100.4 100.0 GBW 04117 产铀岩石 1.0000 0.10 100.4 100.5 99.8 GBW 04119 产铀岩石 1.0000 0.10 99.7 99.8 99.7 GBW 07404 土壤 1.0000 0.10 99.8 100.4 100.3 GBW 07405 土壤 1.0000 0.10 100.3 99.8 100.2 GBW 07310 水系沉积物 1.0000 0.10 100.5 99.7 100.5 GBW 07311 水系沉积物 1.0000 0.10 100.3 99.8 99.9 GBW 04110 铀矿石 0.1000 1.00 100.4 99.6 99.7 GBW 04111 铀矿石 0.1000 1.00 99.7 100.2 100.1 -
[1] Kim C K, Kim C S, Sansone U, Martin P. Development and application of an on-line sequential injection system for the separation of Pu, 210Po and 210Pb from environmental samples[J].Applied Radiation and Isotopes, 2008, 66(2): 223-230. doi: 10.1016/j.apradiso.2007.08.006
[2] Kim C K, Martin P, Fajgelj A. Quantification of mea-surement uncertainty in the sequential determination of 210Pb and 210Po by liquid scintillation counting and alpha-particle spectrometry[J].Accreditation and Quality Assurance, 2008, 13(2): 691-702.
[3] Connan O, Boust D, Billon G, Solier L, Rozet M, Bouderbala S.Solid partitioning and solid-liquid distribution of 210Po and 210Pb in marine anoxic sediments: Roads of Cherbourg at the northwestern France [J].Journal of Environmental Radioactivity,2009, 100(10): 905-913. doi: 10.1016/j.jenvrad.2009.06.016
[4] Yamamoto M, Sakaguchi A, Tomita J, Imanaka T, Shiraishi K. Measurements of 210Po and 210Pb in total diet samples: Estimate of dietary intakes of 210Po and 210Pb for Japanese [J].Journal of Radioanalytical and Nuclear Chemistry, 2009, 279(1): 93-103. doi: 10.1007/s10967-007-7198-8
[5] Kawakami H, Yang Y L, Kusakabe M. Distributions of 210Po and 210Pb radioactivity in the intermediate layer of the northwestern North Pacific [J].Journal of Radioanalytical and Nuclear Chemistry,2009,279(2): 561-566. doi: 10.1007/s10967-008-7324-2
[6] Martin P, Hancock G. Routine analysis of naturally occurring radionuclides in environmental samples by alpha-particle spectrometry[R]. Canberra: Supervising Scientist for the Alligator Rivers Region, 1992.
[7] Desideri D, Guerra F, Meli M A, Testa C. Determin-ation of 210Pb in sediments by extraction chromatography [J].Journal of Radioanalytical and Nuclear Chemistry, 1995,200(5): 385-396. doi: 10.1007/BF02162880
[8] Assunta M M, Desideri D, Roselli C, Feduzi L. Analytical methods to determine 210Po and 210Pb in marine samples [J].Microchemical Journal,2011, 99(2): 273-277. doi: 10.1016/j.microc.2011.05.013
[9] Harada K, Burnett W C, Larock P A. Polonium in Florida groundwater and its possible relationship to the sulfur cycle and bacteria [J]. Geochimica et Cosmo-chimica Acta, 1989,53(1): 143-150. doi: 10.1016/0016-7037(89)90281-0
[10] Claude W S, Conrad P W. Radiochemical determination of lead-210 in uranium ores and air dusts [J].Analytical Chemistry,1977,9(2): 302-306.
[11] Cutshall N H, Larsen I L, Olsen C R. Direct analysis of 210Pb in sediment samples: Self-absorption corrections [J].Nuclear Instruments and Methods, 1983,206(1-2): 309-312. doi: 10.1016/0167-5087(83)91273-5
[12] Villa M, Hurtado S, Manjon G, Garcia-Tenorio R. Calibration and measurement of 210Pb using two independent techniques [J].Radiation Measurements, 2007,42(9): 1552-1560. doi: 10.1016/j.radmeas.2007.05.053
[13] Sima O, Arnold D. Transfer of the efficiency calibration of germanium gamma-ray detectors using the GESPECOR software [J].Applied Radiation and Isotopes, 2002,56(1): 71-75.
[14] Kunzendorf H. A practical approach for self-absorption correction in 210Pb gamma-spectrometric dating [J]. Journal of Radioanalytical and Nuclear Chemistry, 1996,204(1): 23-31. doi: 10.1007/BF02060864
[15] Biggin C D, Cook G T, MacKenzie A B, Pates J M. Time-efficient method for the determination of 210Pb, 210Bi, and 210Po activities in seawater using liquid scintillation spectrometry [J]. Analytical Chemistry, 2002, 74(3): 671-677. doi: 10.1021/ac0107599
[16] 王玉学,郭冬发,黄秋红.镍箔恒温自沉积总α、总β计数法同时或连续测定样品中210Pb、210Bi、210Po[J].铀矿地质,2012,28(3): 165-172.
-



 下载:
下载: